Collagen deposition in a non-fibrotic lung granuloma model after nitric oxide inhibition
- PMID: 9846976
- PMCID: PMC1866342
- DOI: 10.1016/S0002-9440(10)65700-8
Collagen deposition in a non-fibrotic lung granuloma model after nitric oxide inhibition
Abstract
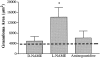
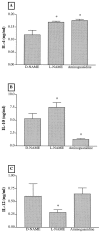
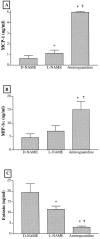
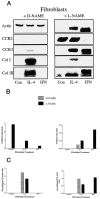
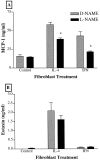
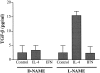
Recent studies support the concept that pulmonary granulomatous inflammation directed by interferon (IFN)-gamma, interleukin (IL)-12, and nitric oxide usually resolves in the absence of fibrosis. To determine whether nitric oxide participates in modulating the fibrotic response during the development of pulmonary granulomas in response to purified protein derivative (PPD), mice presensitized to PPD received daily intraperitoneal injections of N(G)-nitro-D-arginine-methyl ester (D-NAME), N(G)-nitro-L-arginine-methyl ester (L-NAME), or aminoguanidine after delivery of PPD-coated beads to the lungs. Eight days later, morphometric analysis of lung granulomas revealed that L-NAME-treated mice when challenged with PPD in vitro for 36 hours had the largest pulmonary granulomas and the greatest collagen deposition among the treated groups. In addition, equivalent numbers of dispersed lung cells from L-NAME- and aminoguanidine-treated mice produced significantly higher levels of IL-4, monocyte chemoattractant protein (MCP)-1, and macrophage inflammatory protein (MIP)-1alpha and significantly lower levels of eotaxin compared with D-NAME-treated mice. Cultures of dispersed lung cells from L-NAME-treated mice also produced significantly more IL-10 and less IL-12 compared with similar numbers of dispersed lung cells from D-NAME-treated mice. Cultures of isolated lung fibroblasts from L-NAME-treated mice expressed higher levels of C-C chemokine receptor 2 (CCR2) and CCR3 mRNA and contained less MCP-1 and eotaxin protein than a similar number of fibroblasts from D-NAME-treated mice. Thus, nitric oxide appears to regulate the deposition of extracellular matrix in lung granulomas through the modulation of the cytokine and chemokine profile of these lesions. Alterations in the cytokine, chemokine, and procollagen profile of this lesion may be a direct effect of nitric oxide on the pulmonary fibroblast and provide an important signal for regulating fibroblast activity during the evolution of chronic lung disease.
Figures

Similar articles
-
Alteration of the cytokine phenotype in an experimental lung granuloma model by inhibiting nitric oxide.J Immunol. 1997 Dec 1;159(11):5585-93. J Immunol. 1997. PMID: 9548500
-
Differential monocyte chemoattractant protein-1 and chemokine receptor 2 expression by murine lung fibroblasts derived from Th1- and Th2-type pulmonary granuloma models.J Immunol. 1999 Aug 15;163(4):2193-201. J Immunol. 1999. PMID: 10438961
-
Evidence for nitric oxide action on in vitro granuloma formation through pivotal changes in MIP-1alpha and IL-10 release in human schistosomiasis.Nitric Oxide. 1999;3(2):162-71. doi: 10.1006/niox.1999.0219. Nitric Oxide. 1999. PMID: 10369186
-
Nitric oxide is required for effective innate immunity against Klebsiella pneumoniae.Infect Immun. 1997 May;65(5):1870-5. doi: 10.1128/iai.65.5.1870-1875.1997. Infect Immun. 1997. PMID: 9125574 Free PMC article.
-
Therapeutic effects of nitric oxide inhibition during experimental fecal peritonitis: role of interleukin-10 and monocyte chemoattractant protein 1.Infect Immun. 1998 Feb;66(2):650-5. doi: 10.1128/IAI.66.2.650-655.1998. Infect Immun. 1998. PMID: 9453622 Free PMC article.
Cited by
-
Nitric oxide in asthma physiopathology.ISRN Allergy. 2011 Apr 19;2011:832560. doi: 10.5402/2011/832560. Print 2011. ISRN Allergy. 2011. PMID: 23724233 Free PMC article.
-
Effects of nitric oxide synthase inhibitor omega-nitro-L-arginine methyl ester, on silica-induced inflammatory reaction and apoptosis.Part Fibre Toxicol. 2006 Nov 7;3:14. doi: 10.1186/1743-8977-3-14. Part Fibre Toxicol. 2006. PMID: 17090306 Free PMC article.
-
Inducible nitric oxide synthase inhibition influenced granuloma formation with suppressed collagen expression in myositis caused by Toxocara canis in mice.Parasitol Res. 2008 Mar;102(4):577-85. doi: 10.1007/s00436-007-0791-5. Epub 2007 Nov 22. Parasitol Res. 2008. PMID: 18034265
-
Human pulmonary fibroblasts exhibit altered interleukin-4 and interleukin-13 receptor subunit expression in idiopathic interstitial pneumonia.Am J Pathol. 2004 Jun;164(6):1989-2001. doi: 10.1016/S0002-9440(10)63759-5. Am J Pathol. 2004. PMID: 15161635 Free PMC article.
-
IL-12 as a therapeutic target for pharmacological modulation in immune-mediated and inflammatory diseases: regulation of T helper 1/T helper 2 responses.Br J Pharmacol. 1999 Jul;127(6):1295-304. doi: 10.1038/sj.bjp.0702689. Br J Pharmacol. 1999. PMID: 10455278 Free PMC article. Review.
References
-
- Kunkel SL: Th1- and Th2-type cytokines regulate chemokine expression. Biol Signals 1996, 5(4):197-202 - PubMed
-
- Meduri GU, Headley S, Kohler G, Stentz F, Tolley E, Umberger R, Leeper K: Persistent elevation of inflammatory cytokines predicts a poor outcome in ARDS. Plasma IL-1 and IL-6 levels are consistent and efficient predictors of outcome over time. Chest 1995, 107:1062-1073 - PubMed
-
- Schwartz MI: Interstitial pulmonary fibrosis. Kelley WN eds. Textbook of Internal Medicine. 1989, :pp 1902-1905 JP Lippincott, Philadelphia
-
- Chensue SW, Warmington K, Ruth J, Lincoln P, Kuo M-C, Kunkel SL: Cytokine responses during mycobacterial and schistosomal antigen-induced pulmonary granuloma formation: production of Th1 and Th2 cytokines and relative contribution of tumor necrosis factor. Am J Pathol 1994, 145:1105-1113 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

