Chemokines IL-8, GROalpha, MCP-1, IP-10, and Mig are sequentially and differentially expressed during phase-specific infiltration of leukocyte subsets in human wound healing
- PMID: 9846975
- PMCID: PMC1866330
- DOI: 10.1016/s0002-9440(10)65699-4
Chemokines IL-8, GROalpha, MCP-1, IP-10, and Mig are sequentially and differentially expressed during phase-specific infiltration of leukocyte subsets in human wound healing
Abstract
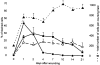
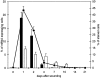
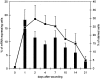
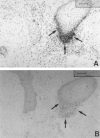
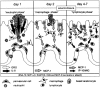
Healing of cutaneous wounds requires a complex integrated network of repair mechanisms, including the action of newly recruited leukocytes. Using a skin repair model in adult humans, we investigated the role chemokines play in sequential infiltration of leukocyte subsets during wound healing. At day 1 after injury, the C-X-C chemokines IL-8 and growth-related oncogene alpha are maximally expressed in the superficial wound bed and are spatially and temporally associated with neutrophil infiltration. IL-8 and growth-related oncogene alpha profiles also correlate with keratinocyte migration and subsequently subside after wound closure at day 4. Macrophage infiltration reaches the highest levels at day 2 and is paralleled by monocyte chemoattractant protein-1 mRNA expression in both the basal layer of the proliferative epidermis at the wound margins and mononuclear cells in the wound area. Other monocyte-attracting chemokines such as monocyte chemoattractant protein-3, macrophage inflammatory protein-1alpha and -1beta, RANTES, and 1309 are undetectable. At day 4, perivascular focal lymphocyte accumulation correlates with strong focal expression of the C-X-C chemokines Mig and IP-10. Our results suggest that a dynamic set of chemokines contributes to the spatially and temporally different infiltration of leukocyte subsets and thus integrates the inflammatory and reparative processes during wound repair.
Figures



Similar articles
-
Differential and sequential expression of multiple chemokines during elicitation of allergic contact hypersensitivity.Am J Pathol. 2001 Feb;158(2):431-40. doi: 10.1016/s0002-9440(10)63986-7. Am J Pathol. 2001. PMID: 11159181 Free PMC article.
-
Accuracy of the Wound Healing Questionnaire in the diagnosis of surgical-site infection after abdominal surgery in low- and middle-income countries.Br J Surg. 2024 Jan 31;111(2):znad446. doi: 10.1093/bjs/znad446. Br J Surg. 2024. PMID: 38747515 Free PMC article. Clinical Trial.
-
Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Message Transmission Between Adipocyte and Macrophage in Obesity.Adv Exp Med Biol. 2024;1460:273-295. doi: 10.1007/978-3-031-63657-8_9. Adv Exp Med Biol. 2024. PMID: 39287855 Review.
Cited by
-
Low-temperature pulverization-specific Sargassum horneri extract accelerates wound healing and attenuates inflammation in a mouse burn model.Anim Cells Syst (Seoul). 2024 Sep 5;28(1):428-438. doi: 10.1080/19768354.2024.2396903. eCollection 2024. Anim Cells Syst (Seoul). 2024. PMID: 39246418 Free PMC article.
-
Involution of the mouse mammary gland is associated with an immune cascade and an acute-phase response, involving LBP, CD14 and STAT3.Breast Cancer Res. 2004;6(2):R75-91. doi: 10.1186/bcr753. Epub 2003 Dec 18. Breast Cancer Res. 2004. PMID: 14979920 Free PMC article.
-
Condomless receptive anal intercourse is associated with markers of mucosal inflammation in a cohort of men who have sex with men in Atlanta, Georgia.J Int AIDS Soc. 2021 Dec;24(12):e25859. doi: 10.1002/jia2.25859. J Int AIDS Soc. 2021. PMID: 34911162 Free PMC article.
-
Alteration of Endothelin 1, MCP-1 and Chromogranin A in patients with atrial fibrillation undergoing pulmonary vein isolation.PLoS One. 2017 Sep 8;12(9):e0184337. doi: 10.1371/journal.pone.0184337. eCollection 2017. PLoS One. 2017. PMID: 28886122 Free PMC article.
-
N-Acetylated Proline-Glycine-Proline Accelerates Cutaneous Wound Healing and Neovascularization by Human Endothelial Progenitor Cells.Sci Rep. 2017 Feb 23;7:43057. doi: 10.1038/srep43057. Sci Rep. 2017. PMID: 28230162 Free PMC article.
References
-
- Clark RAF: Basics of cutaneous wound repair. J Dermatol Surg Oncol 1993, 19:693-706 - PubMed
-
- Clark RAF: The Molecular and Cellular Biology of Wound Repair. 1996. Plenum Press, New York and London
-
- Martin P: Wound healing: aiming for perfect skin regeneration. Science 1997, 276:75-81 - PubMed
-
- Bennett NT, Schultz GS: Growth factors and wound healing. Am J Surg 1993, 165:-737 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

