Separate jasmonate-dependent and salicylate-dependent defense-response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens
- PMID: 9844023
- PMCID: PMC24583
- DOI: 10.1073/pnas.95.25.15107
Separate jasmonate-dependent and salicylate-dependent defense-response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens
Abstract
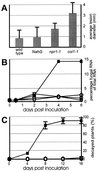
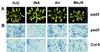
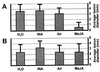
The endogenous plant hormones salicylic acid (SA) and jasmonic acid (JA), whose levels increase on pathogen infection, activate separate sets of genes encoding antimicrobial proteins in Arabidopsis thaliana. The pathogen-inducible genes PR-1, PR-2, and PR-5 require SA signaling for activation, whereas the plant defensin gene PDF1.2, along with a PR-3 and PR-4 gene, are induced by pathogens via an SA-independent and JA-dependent pathway. An Arabidopsis mutant, coi1, that is affected in the JA-response pathway shows enhanced susceptibility to infection by the fungal pathogens Alternaria brassicicola and Botrytis cinerea but not to Peronospora parasitica, and vice versa for two Arabidopsis genotypes (npr1 and NahG) with a defect in their SA response. Resistance to P. parasitica was boosted by external application of the SA-mimicking compound 2, 6-dichloroisonicotinic acid [Delaney, T., et al. (1994) Science 266, 1247-1250] but not by methyl jasmonate (MeJA), whereas treatment with MeJA but not 2,6-dichloroisonicotinic acid elevated resistance to Alternaria brassicicola. The protective effect of MeJA against A. brassicicola was the result of an endogenous defense response activated in planta and not a direct effect of MeJA on the pathogen, as no protection to A. brassicicola was observed in the coi1 mutant treated with MeJA. These data point to the existence of at least two separate hormone-dependent defense pathways in Arabidopsis that contribute to resistance against distinct microbial pathogens.
Figures


Similar articles
-
Ethylene and jasmonic acid signaling affect the NPR1-independent expression of defense genes without impacting resistance to Pseudomonas syringae and Peronospora parasitica in the Arabidopsis ssi1 mutant.Mol Plant Microbe Interact. 2003 Jul;16(7):588-99. doi: 10.1094/MPMI.2003.16.7.588. Mol Plant Microbe Interact. 2003. PMID: 12848424
-
The BOS loci of Arabidopsis are required for resistance to Botrytis cinerea infection.Plant J. 2004 Nov;40(4):558-74. doi: 10.1111/j.1365-313X.2004.02232.x. Plant J. 2004. PMID: 15500471
-
Differential effectiveness of salicylate-dependent and jasmonate/ethylene-dependent induced resistance in Arabidopsis.Mol Plant Microbe Interact. 2002 Jan;15(1):27-34. doi: 10.1094/MPMI.2002.15.1.27. Mol Plant Microbe Interact. 2002. PMID: 11858171
-
Early responses in the Arabidopsis-Verticillium longisporum pathosystem are dependent on NDR1, JA- and ET-associated signals via cytosolic NPR1 and RFO1.Mol Plant Microbe Interact. 2006 Sep;19(9):958-69. doi: 10.1094/MPMI-19-0958. Mol Plant Microbe Interact. 2006. PMID: 16941900
-
Comparison of the pathway structures influencing the temporal response of salicylate and jasmonate defence hormones in Arabidopsis thaliana.Front Plant Sci. 2022 Sep 9;13:952301. doi: 10.3389/fpls.2022.952301. eCollection 2022. Front Plant Sci. 2022. PMID: 36160984 Free PMC article. Review.
Cited by
-
PtrWRKY73, a salicylic acid-inducible poplar WRKY transcription factor, is involved in disease resistance in Arabidopsis thaliana.Plant Cell Rep. 2015 May;34(5):831-41. doi: 10.1007/s00299-015-1745-5. Epub 2015 Jan 28. Plant Cell Rep. 2015. PMID: 25627252 Free PMC article.
-
Over-expression of TaMYB33 encoding a novel wheat MYB transcription factor increases salt and drought tolerance in Arabidopsis.Mol Biol Rep. 2012 Jun;39(6):7183-92. doi: 10.1007/s11033-012-1550-y. Epub 2012 Feb 17. Mol Biol Rep. 2012. PMID: 22350156
-
EST sequencing and gene expression profiling of defence-related genes from Persea americana infected with Phytophthora cinnamomi.BMC Plant Biol. 2011 Nov 23;11:167. doi: 10.1186/1471-2229-11-167. BMC Plant Biol. 2011. PMID: 22108245 Free PMC article.
-
Identification and Characterization of High-Molecular-Weight Proteins Secreted by Plasmodiophora brassicae That Suppress Plant Immunity.J Fungi (Basel). 2024 Jun 29;10(7):462. doi: 10.3390/jof10070462. J Fungi (Basel). 2024. PMID: 39057347 Free PMC article.
-
Yeast cell wall extract induces disease resistance against bacterial and fungal pathogens in Arabidopsis thaliana and Brassica crop.PLoS One. 2015 Jan 7;10(1):e0115864. doi: 10.1371/journal.pone.0115864. eCollection 2015. PLoS One. 2015. PMID: 25565273 Free PMC article.
References
-
- Dürner J, Shah J, Klessig D F. Trends Plant Sci. 1997;2:266–274.
-
- Uknes S, Winter A, Delaney T, Vernooij B, Morse A, Friedrich L, Nye G, Potter S, Ward E, Ryals J A. Mol Plant–Microbe Interact. 1993;6:692–698.
-
- Delaney T, Uknes S, Vernooij B, Friedrich L, Weymann K, Negrotto D, Gaffney T, Gut-Rella M, Kessmann H, Ward E, et al. Science. 1994;266:1247–1250. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

