Suppression of protein synthesis in brain during hibernation involves inhibition of protein initiation and elongation
- PMID: 9826731
- PMCID: PMC24404
- DOI: 10.1073/pnas.95.24.14511
Suppression of protein synthesis in brain during hibernation involves inhibition of protein initiation and elongation
Abstract
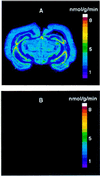
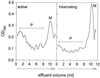
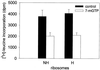
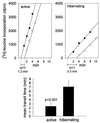
Protein synthesis (PS) has been considered essential to sustain mammalian life, yet was found to be virtually arrested for weeks in brain and other organs of the hibernating ground squirrel, Spermophilus tridecemlineatus. PS, in vivo, was below the limit of autoradiographic detection in brain sections and, in brain extracts, was determined to be 0.04% of the average rate from active squirrels. Further, it was reduced 3-fold in cell-free extracts from hibernating brain at 37 degreesC, eliminating hypothermia as the only cause for protein synthesis inhibition (active, 0.47 +/- 0.08 pmol/mg protein per min; hibernator, 0.16 +/- 0.05 pmol/mg protein per min, P < 0.001). PS suppression involved blocks of initiation and elongation, and its onset coincided with the early transition phase into hibernation. An increased monosome peak with moderate ribosomal disaggregation in polysome profiles and the greatly increased phosphorylation of eIF2alpha are both consistent with an initiation block in hibernators. The elongation block was demonstrated by a 3-fold increase in ribosomal mean transit times in cell-free extracts from hibernators (active, 2.4 +/- 0.7 min; hibernator, 7.1 +/- 1.4 min, P < 0.001). No abnormalities of ribosomal function or mRNA levels were detected. These findings implicate suppression of PS as a component of the regulated shutdown of cellular function that permits hibernating ground squirrels to tolerate "trickle" blood flow and reduced substrate and oxygen availability. Further study of the factors that control these phenomena may lead to identification of the molecular mechanisms that regulate this state.
Figures



Similar articles
-
Mechanisms for increased levels of phosphorylation of elongation factor-2 during hibernation in ground squirrels.Biochemistry. 2001 Sep 25;40(38):11565-70. doi: 10.1021/bi010649w. Biochemistry. 2001. PMID: 11560506
-
Translational initiation is uncoupled from elongation at 18 degrees C during mammalian hibernation.Am J Physiol Regul Integr Comp Physiol. 2001 Nov;281(5):R1374-9. doi: 10.1152/ajpregu.2001.281.5.R1374. Am J Physiol Regul Integr Comp Physiol. 2001. PMID: 11641105
-
The translation state of differentially expressed mRNAs in the hibernating 13-lined ground squirrel (Spermophilus tridecemlineatus).Arch Biochem Biophys. 2002 May 15;401(2):244-54. doi: 10.1016/S0003-9861(02)00048-6. Arch Biochem Biophys. 2002. PMID: 12054475
-
Mammalian hibernation. Transcriptional and translational controls.Adv Exp Med Biol. 2003;543:21-38. Adv Exp Med Biol. 2003. PMID: 14713112 Review.
-
Eukaryotic protein synthesis.Annu Rev Biochem. 1985;54:1109-49. doi: 10.1146/annurev.bi.54.070185.005333. Annu Rev Biochem. 1985. PMID: 3896117 Review. No abstract available.
Cited by
-
Regulation of Torpor in the Gray Mouse Lemur: Transcriptional and Translational Controls and Role of AMPK Signaling.Genomics Proteomics Bioinformatics. 2015 Apr;13(2):103-10. doi: 10.1016/j.gpb.2015.03.003. Epub 2015 Jun 17. Genomics Proteomics Bioinformatics. 2015. PMID: 26092186 Free PMC article.
-
Rapid and reversible changes in intrahippocampal connectivity during the course of hibernation in European hamsters.Proc Natl Acad Sci U S A. 2006 Dec 5;103(49):18775-80. doi: 10.1073/pnas.0608785103. Epub 2006 Nov 22. Proc Natl Acad Sci U S A. 2006. PMID: 17121986 Free PMC article.
-
Dynamic temperature-sensitive A-to-I RNA editing in the brain of a heterothermic mammal during hibernation.RNA. 2018 Nov;24(11):1481-1495. doi: 10.1261/rna.066522.118. Epub 2018 Jul 31. RNA. 2018. PMID: 30065024 Free PMC article.
-
Up-regulation of Long Non-coding RNA TUG1 in Hibernating Thirteen-lined Ground Squirrels.Genomics Proteomics Bioinformatics. 2016 Apr;14(2):113-8. doi: 10.1016/j.gpb.2016.03.004. Epub 2016 Apr 27. Genomics Proteomics Bioinformatics. 2016. PMID: 27132145 Free PMC article.
-
Reversible suppression of protein synthesis in concert with polysome disaggregation during anoxia exposure in Littorina littorea.Mol Cell Biochem. 2002 Mar;232(1-2):121-7. doi: 10.1023/a:1014811017753. Mol Cell Biochem. 2002. PMID: 12030368
References
-
- Frerichs K U, Kennedy C, Sokoloff L, Hallenbeck J M. J Cereb Blood Flow Metab. 1994;14:193–205. - PubMed
-
- Astrup J, Siesjo B K, Symon L. Stroke. 1981;12:723–725. - PubMed
-
- Frerichs K U, Hallenbeck J M. J Cereb Blood Flow Metab. 1998;18:168–175. - PubMed
-
- Storey K B. Comp Biochem Physiol. 1996;113B:23–35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

