Nitrosative stress: metabolic pathway involving the flavohemoglobin
- PMID: 9826660
- PMCID: PMC24333
- DOI: 10.1073/pnas.95.24.14100
Nitrosative stress: metabolic pathway involving the flavohemoglobin
Abstract
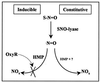
Nitric oxide (NO) biology has focused on the tightly regulated enzymatic mechanism that transforms L-arginine into a family of molecules, which serve both signaling and defense functions. However, very little is known of the pathways that metabolize these molecules or turn off the signals. The paradigm is well exemplified in bacteria where S-nitrosothiols (SNO)-compounds identified with antimicrobial activities of NO synthase-elicit responses that mediate bacterial resistance by unknown mechanisms. Here we show that Escherichia coli possess both constitutive and inducible elements for SNO metabolism. Constitutive enzyme(s) cleave SNO to NO whereas bacterial hemoglobin, a widely distributed flavohemoglobin of poorly understood function, is central to the inducible response. Remarkably, the protein has evolved a novel heme-detoxification mechanism for NO. Specifically, the heme serves a dioxygenase function that produces mainly nitrate. These studies thus provide new insights into SNO and NO metabolism and identify enzymes with reactions that were thought to occur only by chemical means. Our results also emphasize that the reactions of SNO and NO with hemoglobins are evolutionary conserved, but have been adapted for cell-specific function.
Figures
Similar articles
-
Nitric oxide-mediated heme oxidation and selective beta-globin nitrosation of hemoglobin from normal and sickle erythrocytes.Biochem Biophys Res Commun. 2000 Sep 7;275(3):962-7. doi: 10.1006/bbrc.2000.3413. Biochem Biophys Res Commun. 2000. PMID: 10973828
-
Nitric oxide and thiol groups.Biochim Biophys Acta. 1999 May 5;1411(2-3):323-33. doi: 10.1016/s0005-2728(99)00023-7. Biochim Biophys Acta. 1999. PMID: 10320666 Review.
-
Structure-reactivity studies of the Cu(2+)-catalyzed decomposition of four S-nitrosothiols based around the S-Nitrosocysteine/S-nitrosoglutathione structures.Nitric Oxide. 2000 Aug;4(4):392-8. doi: 10.1006/niox.2000.0291. Nitric Oxide. 2000. PMID: 10944424
-
A metabolic enzyme for S-nitrosothiol conserved from bacteria to humans.Nature. 2001 Mar 22;410(6827):490-4. doi: 10.1038/35068596. Nature. 2001. PMID: 11260719
-
Nitric oxide, S-nitrosylation and neurodegeneration.Cell Mol Biol (Noisy-le-grand). 2005 Sep 5;51(3):247-54. Cell Mol Biol (Noisy-le-grand). 2005. PMID: 16191392 Review.
Cited by
-
Biological Mechanisms of S-Nitrosothiol Formation and Degradation: How Is Specificity of S-Nitrosylation Achieved?Antioxidants (Basel). 2021 Jul 12;10(7):1111. doi: 10.3390/antiox10071111. Antioxidants (Basel). 2021. PMID: 34356344 Free PMC article. Review.
-
Transcriptomic Profiling of High-Density Giardia Foci Encysting in the Murine Proximal Intestine.Front Cell Infect Microbiol. 2017 May 31;7:227. doi: 10.3389/fcimb.2017.00227. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28620589 Free PMC article.
-
NO sensing by FNR: regulation of the Escherichia coli NO-detoxifying flavohaemoglobin, Hmp.EMBO J. 2002 Jul 1;21(13):3235-44. doi: 10.1093/emboj/cdf339. EMBO J. 2002. PMID: 12093725 Free PMC article.
-
Construction and Experimental Validation of a Quantitative Kinetic Model of Nitric Oxide Stress in Enterohemorrhagic Escherichia coli O157:H7.Bioengineering (Basel). 2016 Feb 6;3(1):9. doi: 10.3390/bioengineering3010009. Bioengineering (Basel). 2016. PMID: 28952571 Free PMC article.
-
Essential role of flavohemoglobin in long-term anaerobic survival of Bacillus subtilis.J Bacteriol. 2006 Sep;188(17):6415-8. doi: 10.1128/JB.00557-06. J Bacteriol. 2006. PMID: 16923910 Free PMC article.
References
-
- Nathan C. FASEB J. 1992;6:3051–3064. - PubMed
-
- Xu L, Eu J P, Meissner G, Stamler J S. Science. 1998;279:234–237. - PubMed
-
- Lipton S A, Choi Y B, Pan Z H, Lei S Z, Chen H S, Sucher N J, Loscalzo J, Singel D J, Stamler J S. Nature (London) 1993;364:626–632. - PubMed
-
- Lander H M, Ogiste J S, Teng K K, Novogrodsky A. J Biol Chem. 1995;270:21195–21198. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

