Subcellular redistribution of m2 muscarinic acetylcholine receptors in striatal interneurons in vivo after acute cholinergic stimulation
- PMID: 9822774
- PMCID: PMC6793283
- DOI: 10.1523/JNEUROSCI.18-23-10207.1998
Subcellular redistribution of m2 muscarinic acetylcholine receptors in striatal interneurons in vivo after acute cholinergic stimulation
Abstract
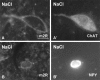
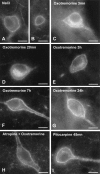
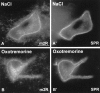
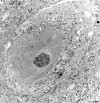
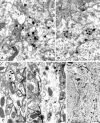
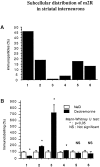
The purpose of our work was to investigate how the cholinergic environment influences the targeting and the intracellular trafficking of the muscarinic receptor m2 (m2R) in vivo. To address this question, we have used immunohistochemical approaches at light and electron microscopic levels to detect the m2R in control rats and rats treated with muscarinic receptor agonists. In control animals, m2Rs were located mostly at postsynaptic sites at the plasma membrane of perikarya and dendrites of cholinergic and NPY-somatostatin interneurons as autoreceptors and heteroreceptors, respectively. Presynaptic receptors were also detected in boutons. The m2Rs were usually detected at extrasynaptic sites, but they could be found rarely in association with symmetrical synapses, suggesting that the cholinergic transmission mediated by m2R occurs via synaptic and nonsynaptic mechanisms. The stimulation of muscarinic receptors with oxotremorine provoked a dramatic alteration of m2R compartmentalization, including endocytosis with a decrease of the density of m2R at the membrane (-63%) and an increase of those associated with endosomes (+86%) in perikarya. The very strong increase of m2R associated with multivesicular bodies (+732%) suggests that oxotremorine activated degradation. The slight increase in the Golgi apparatus (+26%) suggests that the m2R stimulation had an effect on the maturation of m2R. The substance P receptor located at the membrane of the same neurons was unaffected by oxotremorine. Our data demonstrate that cholinergic stimulation dramatically influences the subcellular distribution of m2R in striatal interneurons in vivo. These events may have key roles in controlling abundance and availability of muscarinic receptors via regulation of receptor endocytosis, degradation, and/or neosynthesis. Further, the control of muscarinic receptor trafficking may influence the activity of striatal interneurons, including neurotransmitter release and/or electric activity.
Figures

Similar articles
-
Regulation of the subcellular distribution of m4 muscarinic acetylcholine receptors in striatal neurons in vivo by the cholinergic environment: evidence for regulation of cell surface receptors by endogenous and exogenous stimulation.J Neurosci. 1999 Dec 1;19(23):10237-49. doi: 10.1523/JNEUROSCI.19-23-10237.1999. J Neurosci. 1999. PMID: 10575021 Free PMC article.
-
Trafficking of the muscarinic m2 autoreceptor in cholinergic basalocortical neurons in vivo: differential regulation of plasma membrane receptor availability and intraneuronal localization in acetylcholinesterase-deficient and -inhibited mice.J Comp Neurol. 2003 Jun 9;462(3):302-14. doi: 10.1002/cne.10734. J Comp Neurol. 2003. PMID: 12794734
-
Evidence for M2 muscarinic receptor modulation of axon terminals and dendrites in the rodent basolateral amygdala: An ultrastructural and electrophysiological analysis.Neuroscience. 2017 Aug 15;357:349-362. doi: 10.1016/j.neuroscience.2017.06.019. Epub 2017 Jun 17. Neuroscience. 2017. PMID: 28629847 Free PMC article.
-
"In vivo" intraneuronal trafficking of G protein coupled receptors in the striatum: regulation by dopaminergic and cholinergic environment.Biol Cell. 2003 Oct;95(7):477-88. doi: 10.1016/s0248-4900(03)00080-7. Biol Cell. 2003. PMID: 14597266 Review.
-
Cholinergic modulation of striatal microcircuits.Eur J Neurosci. 2019 Mar;49(5):604-622. doi: 10.1111/ejn.13949. Epub 2018 Nov 29. Eur J Neurosci. 2019. PMID: 29797362 Free PMC article. Review.
Cited by
-
Dopamine tone regulates D1 receptor trafficking and delivery in striatal neurons in dopamine transporter-deficient mice.Proc Natl Acad Sci U S A. 2000 Feb 15;97(4):1879-84. doi: 10.1073/pnas.97.4.1879. Proc Natl Acad Sci U S A. 2000. PMID: 10677550 Free PMC article.
-
The thalamostriatal pathway and cholinergic control of goal-directed action: interlacing new with existing learning in the striatum.Neuron. 2013 Jul 10;79(1):153-66. doi: 10.1016/j.neuron.2013.04.039. Epub 2013 Jun 13. Neuron. 2013. PMID: 23770257 Free PMC article.
-
Striatal cholinergic dysfunction as a unifying theme in the pathophysiology of dystonia.Prog Neurobiol. 2015 Apr;127-128:91-107. doi: 10.1016/j.pneurobio.2015.02.002. Epub 2015 Feb 17. Prog Neurobiol. 2015. PMID: 25697043 Free PMC article. Review.
-
Acetylcholine-dopamine interactions in the pathophysiology and treatment of CNS disorders.CNS Neurosci Ther. 2010 Jun;16(3):137-62. doi: 10.1111/j.1755-5949.2010.00142.x. Epub 2010 Mar 29. CNS Neurosci Ther. 2010. PMID: 20370804 Free PMC article. Review.
-
Differential subcellular localization of mGluR1a and mGluR5 in the rat and monkey Substantia nigra.J Neurosci. 2001 Mar 15;21(6):1838-47. doi: 10.1523/JNEUROSCI.21-06-01838.2001. J Neurosci. 2001. PMID: 11245668 Free PMC article.
References
-
- Barnes PJ, Haddad EB, Rousell J. Regulation of muscarinic M2 receptors. Life Sci. 1997;60:1015–1021. - PubMed
-
- Baude A, Nusser Z, Molnar E, McIlhinney RAJ, Somogyi P. High-resolution immunogold localization of AMPA type glutamate receptor subunits at synaptic and non-synaptic sites in rat hippocampus. Neuroscience. 1995;69:1031–1055. - PubMed
-
- Bernard V, Dumartin B, Lamy E, Bloch B. Fos immunoreactivity after stimulation or inhibition of muscarinic receptors indicates anatomical specificity for cholinergic control of striatal efferent neurons and cortical neurons in rat. Eur J Neurosci. 1993;5:1218–1225. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
