Alzheimer amyloid protein precursor in the rat hippocampus: transport and processing through the perforant path
- PMID: 9822724
- PMCID: PMC6793291
- DOI: 10.1523/JNEUROSCI.18-23-09629.1998
Alzheimer amyloid protein precursor in the rat hippocampus: transport and processing through the perforant path
Abstract
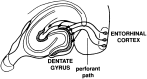
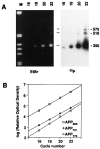
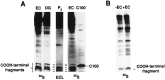
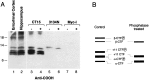
Amyloid deposition is a neuropathological hallmark of Alzheimer's disease. The principal component of amyloid deposits is beta amyloid peptide (Abeta), a peptide derived by proteolytic processing of the amyloid precursor protein (APP). APP is axonally transported by the fast anterograde component. Several studies have indicated that Abeta deposits occur in proximity to neuritic and synaptic profiles. Taken together, these latter observations have suggested that APP, axonally transported to nerve terminals, may be processed to Abeta at those sites. To examine the fate of APP in the CNS, we injected [35S]methionine into the rat entorhinal cortex and examined the trafficking and processing of de novo synthesized APP in the perforant pathway and at presynaptic sites in the hippocampal formation. We report that both full-length and processed APP accumulate at presynaptic terminals of entorhinal neurons. Finally, we demonstrate that at these synaptic sites, C-terminal fragments of APP containing the entire Abeta domain accumulate, suggesting that these species may represent the penultimate precursors of synaptic Abeta.
Figures

Similar articles
-
Evidence that synaptically released beta-amyloid accumulates as extracellular deposits in the hippocampus of transgenic mice.J Neurosci. 2002 Nov 15;22(22):9785-93. doi: 10.1523/JNEUROSCI.22-22-09785.2002. J Neurosci. 2002. PMID: 12427834 Free PMC article.
-
Disruption of corticocortical connections ameliorates amyloid burden in terminal fields in a transgenic model of Abeta amyloidosis.J Neurosci. 2002 Nov 15;22(22):9794-9. doi: 10.1523/JNEUROSCI.22-22-09794.2002. J Neurosci. 2002. PMID: 12427835 Free PMC article.
-
Regulation of Synaptic Amyloid-β Generation through BACE1 Retrograde Transport in a Mouse Model of Alzheimer's Disease.J Neurosci. 2017 Mar 8;37(10):2639-2655. doi: 10.1523/JNEUROSCI.2851-16.2017. Epub 2017 Feb 3. J Neurosci. 2017. PMID: 28159908 Free PMC article.
-
Subcellular trafficking of the amyloid precursor protein gene family and its pathogenic role in Alzheimer's disease.Neurodegener Dis. 2006;3(4-5):218-26. doi: 10.1159/000095259. Neurodegener Dis. 2006. PMID: 17047360 Review.
-
Alzheimer's disease.Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
Cited by
-
Quantification of amyloid precursor protein and tau for the study of axonal traffic pathways.J Neurosci. 2007 Mar 28;27(13):3357-63. doi: 10.1523/JNEUROSCI.5024-06.2007. J Neurosci. 2007. PMID: 17392450 Free PMC article. No abstract available.
-
Aberrant subcellular neuronal calcium regulation in aging and Alzheimer's disease.Biochim Biophys Acta. 2011 May;1813(5):965-73. doi: 10.1016/j.bbamcr.2010.10.005. Epub 2010 Oct 13. Biochim Biophys Acta. 2011. PMID: 20950656 Free PMC article. Review.
-
Genetically engineered models relevant to neurodegenerative disorders: their value for understanding disease mechanisms and designing/testing experimental therapeutics.J Mol Neurosci. 2001 Oct;17(2):233-57. doi: 10.1385/JMN:17:2:233. J Mol Neurosci. 2001. PMID: 11816796 Review.
-
Transsynaptic progression of amyloid-β-induced neuronal dysfunction within the entorhinal-hippocampal network.Neuron. 2010 Nov 4;68(3):428-41. doi: 10.1016/j.neuron.2010.10.020. Neuron. 2010. PMID: 21040845 Free PMC article.
-
Identification of NEEP21 as a ß-amyloid precursor protein-interacting protein in vivo that modulates amyloidogenic processing in vitro.J Neurosci. 2010 Nov 17;30(46):15677-85. doi: 10.1523/JNEUROSCI.4464-10.2010. J Neurosci. 2010. PMID: 21084623 Free PMC article.
References
-
- Abe K, Tanzi RE, Kogure K. Selective induction of Kunitz-type protease inhibitor domain-containing amyloid precursor protein mRNA after persistent focal ischemia in rat cerebral cortex. Neurosci Lett. 1991;124:172–174. - PubMed
-
- Arnold SE, Hyman BT, Flory J, Damasio AR, Van Hoesen GW. The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer’s disease. Cereb Cortex. 1991;1:103–116. - PubMed
-
- Banati RB, Gehrmann J, Czech C, Monning U, Jones LL, Konig G, Beyreuther K, Kreutzberg GW. Early and rapid de novo synthesis of Alzheimer βA4-amyloid precursor protein (APP) in activated microglia. Glia. 1993;9:199–210. - PubMed
-
- Beyreuther K, Masters CL. Amyloid precursor protein (APP) and βA4 amyloid in the etiology of Alzheimer’s disease: precursor–product relationships in the derangement of neuronal function. Brain Pathol. 1991;1:241–251. - PubMed
-
- Braak H, Braak E. Pathology of Alzheimer’s disease. In: Calne DB, editor. Neurodegenerative diseases. Saunders; Philadelphia: 1994. pp. 585–613.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
