Identification and characterization of pancreatic eukaryotic initiation factor 2 alpha-subunit kinase, PEK, involved in translational control
- PMID: 9819435
- PMCID: PMC109330
- DOI: 10.1128/MCB.18.12.7499
Identification and characterization of pancreatic eukaryotic initiation factor 2 alpha-subunit kinase, PEK, involved in translational control
Abstract
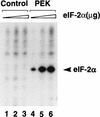
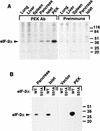
In response to various environmental stresses, eukaryotic cells down-regulate protein synthesis by phosphorylation of the alpha subunit of eukaryotic translation initiation factor 2 (eIF-2alpha). In mammals, the phosphorylation was shown to be carried out by eIF-2alpha kinases PKR and HRI. We report the identification and characterization of a cDNA from rat pancreatic islet cells that encodes a new related kinase, which we term pancreatic eIF-2alpha kinase, or PEK. In addition to a catalytic domain with sequence and structural features conserved among eIF-2alpha kinases, PEK contains a distinctive amino-terminal region 550 residues in length. Using recombinant PEK produced in Escherichia coli or Sf-9 insect cells, we demonstrate that PEK is autophosphorylated on both serine and threonine residues and that the recombinant enzyme can specifically phosphorylate eIF-2alpha on serine-51. Northern blot analyses indicate that PEK mRNA is expressed in all tissues examined, with highest levels in pancreas cells. Consistent with our mRNA assays, PEK activity was predominantly detected in pancreas and pancreatic islet cells. The regulatory role of PEK in protein synthesis was demonstrated both in vitro and in vivo. The addition of recombinant PEK to reticulocyte lysates caused a dose-dependent inhibition of translation. In the Saccharomyces model system, PEK functionally substituted for the endogenous yeast eIF-2alpha kinase, GCN2, by a process requiring the serine-51 phosphorylation site in eIF-2alpha. We also identified PEK homologs from both Caenorhabditis elegans and the puffer fish Fugu rubripes, suggesting that this eIF-2alpha kinase plays an important role in translational control from nematodes to mammals.
Figures



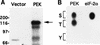

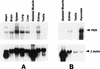


Similar articles
-
Characterization of a mutant pancreatic eIF-2alpha kinase, PEK, and co-localization with somatostatin in islet delta cells.J Biol Chem. 1999 Feb 26;274(9):5723-30. doi: 10.1074/jbc.274.9.5723. J Biol Chem. 1999. PMID: 10026192
-
Pancreatic eukaryotic initiation factor-2alpha kinase (PEK) homologues in humans, Drosophila melanogaster and Caenorhabditis elegans that mediate translational control in response to endoplasmic reticulum stress.Biochem J. 2000 Mar 1;346 Pt 2(Pt 2):281-93. Biochem J. 2000. PMID: 10677345 Free PMC article.
-
A mammalian homologue of GCN2 protein kinase important for translational control by phosphorylation of eukaryotic initiation factor-2alpha.Genetics. 2000 Feb;154(2):787-801. doi: 10.1093/genetics/154.2.787. Genetics. 2000. PMID: 10655230 Free PMC article.
-
The eIF-2alpha kinases and the control of protein synthesis.FASEB J. 1996 Oct;10(12):1378-87. doi: 10.1096/fasebj.10.12.8903508. FASEB J. 1996. PMID: 8903508 Review.
-
Small molecule modulators of eukaryotic initiation factor 2α kinases, the key regulators of protein synthesis.Biochimie. 2013 Nov;95(11):1980-90. doi: 10.1016/j.biochi.2013.07.030. Epub 2013 Aug 11. Biochimie. 2013. PMID: 23939221 Review.
Cited by
-
PERK Regulates Working Memory and Protein Synthesis-Dependent Memory Flexibility.PLoS One. 2016 Sep 14;11(9):e0162766. doi: 10.1371/journal.pone.0162766. eCollection 2016. PLoS One. 2016. PMID: 27627766 Free PMC article.
-
The cellular response to protein misfolding in the endoplasmic reticulum.Gene Expr. 1999;7(4-6):293-300. Gene Expr. 1999. PMID: 10440230 Free PMC article. Review.
-
Endoplasmic reticulum stress: cell life and death decisions.J Clin Invest. 2005 Oct;115(10):2656-64. doi: 10.1172/JCI26373. J Clin Invest. 2005. PMID: 16200199 Free PMC article. Review.
-
Endoplasmic reticulum stress response in cancer: molecular mechanism and therapeutic potential.Am J Transl Res. 2010 Jan 1;2(1):65-74. Am J Transl Res. 2010. PMID: 20182583 Free PMC article.
-
mRNA translation is compartmentalized to the endoplasmic reticulum following physiological inhibition of cap-dependent translation.RNA. 2006 May;12(5):775-89. doi: 10.1261/rna.2318906. Epub 2006 Mar 15. RNA. 2006. PMID: 16540694 Free PMC article.
References
-
- Barber G N, Jagus R, Meurs E F, Hovanessian A G, Katze M G. Molecular mechanisms responsible for malignant transformation by regulatory and catalytic domain variants of the interferon-induced enzyme RNA-dependent protein kinase. J Biol Chem. 1995;270:17423–17428. - PubMed
-
- Bonner-Weir S, Smith F E. Islets of langerhans: morphology and its implications. In: Kahn C R, Weir G C, editors. Joslin’s diabetes mellitus. 13th ed. Piladelphia, Pa: Lea & Febiger; 1994. pp. 15–28.
-
- Cesareni G, Murray J. Plasmid vectors carrying the replication origin of filamentous single-stranded phages. In: Setlow J K, Hollaender A, editors. Genetic engineering: principles and methods. Vol. 9. New York, N.Y: Plenum Press; 1987. pp. 135–154.
-
- Chen J J, London I M. Regulation of protein synthesis by heme-regulated eIF-2 alpha kinase. Trends Biochem Sci. 1995;20:105–108. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
