NF-kappaB activation provides the potential link between inflammation and hyperplasia in the arthritic joint
- PMID: 9811891
- PMCID: PMC24931
- DOI: 10.1073/pnas.95.23.13859
NF-kappaB activation provides the potential link between inflammation and hyperplasia in the arthritic joint
Abstract
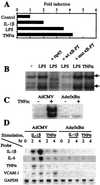
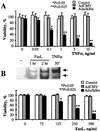
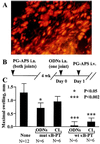
The transcription factor NF-kappaB is a pivotal regulator of inflammatory responses. While the activation of NF-kappaB in the arthritic joint has been associated with rheumatoid arthritis (RA), its significance is poorly understood. Here, we examine the role of NF-kappaB in animal models of RA. We demonstrate that in vitro, NF-kappaB controlled expression of numerous inflammatory molecules in synoviocytes and protected cells against tumor necrosis factor alpha (TNFalpha) and Fas ligand (FasL) cytotoxicity. Similar to that observed in human RA, NF-kappaB was found to be activated in the synovium of rats with streptococcal cell wall (SCW)-induced arthritis. In vivo suppression of NF-kappaB by either proteasomal inhibitors or intraarticular adenoviral gene transfer of super-repressor IkappaBalpha profoundly enhanced apoptosis in the synovium of rats with SCW- and pristane-induced arthritis. This indicated that the activation of NF-kappaB protected the cells in the synovium against apoptosis and thus provided the potential link between inflammation and hyperplasia. Intraarticular administration of NF-kB decoys prevented the recurrence of SCW arthritis in treated joints. Unexpectedly, the severity of arthritis also was inhibited significantly in the contralateral, untreated joints, indicating beneficial systemic effects of local suppression of NF-kappaB. These results establish a mechanism regulating apoptosis in the arthritic joint and indicate the feasibility of therapeutic approaches to RA based on the specific suppression of NF-kappaB.
Figures


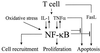
Similar articles
-
The suppressive effects of Saposhnikovia divaricata (Fangfeng) chromone extract on rheumatoid arthritis via inhibition of nuclear factor-κB and mitogen activated proteinkinases activation on collagen-induced arthritis model.J Ethnopharmacol. 2013 Jul 30;148(3):842-50. doi: 10.1016/j.jep.2013.05.023. Epub 2013 May 25. J Ethnopharmacol. 2013. PMID: 23711830
-
CYLD suppression enhances the pro-inflammatory effects and hyperproliferation of rheumatoid arthritis fibroblast-like synoviocytes by enhancing NF-κB activation.Arthritis Res Ther. 2018 Oct 3;20(1):219. doi: 10.1186/s13075-018-1722-9. Arthritis Res Ther. 2018. PMID: 30285829 Free PMC article.
-
NF-kappa B in rheumatoid arthritis: a pivotal regulator of inflammation, hyperplasia, and tissue destruction.Arthritis Res. 2001;3(4):200-6. doi: 10.1186/ar300. Epub 2001 Mar 26. Arthritis Res. 2001. PMID: 11438035 Free PMC article.
-
Nuclear factor-κB in rheumatoid arthritis.Int J Rheum Dis. 2020 Dec;23(12):1627-1635. doi: 10.1111/1756-185X.13958. Epub 2020 Sep 23. Int J Rheum Dis. 2020. PMID: 32965792 Review.
-
The p53 status in rheumatoid arthritis with focus on fibroblast-like synoviocytes.Immunol Res. 2021 Jun;69(3):225-238. doi: 10.1007/s12026-021-09202-7. Epub 2021 May 13. Immunol Res. 2021. PMID: 33983569 Review.
Cited by
-
SKLB023 blocks joint inflammation and cartilage destruction in arthritis models via suppression of nuclear factor-kappa B activation in macrophage.PLoS One. 2013;8(2):e56349. doi: 10.1371/journal.pone.0056349. Epub 2013 Feb 19. PLoS One. 2013. PMID: 23431370 Free PMC article.
-
Use of cell permeable NBD peptides for suppression of inflammation.Ann Rheum Dis. 2006 Nov;65 Suppl 3(Suppl 3):iii75-82. doi: 10.1136/ard.2006.058438. Ann Rheum Dis. 2006. PMID: 17038479 Free PMC article. Review.
-
Current perspectives on synovitis.Arthritis Res. 1999;1(1):11-6. doi: 10.1186/ar4. Epub 1999 Oct 26. Arthritis Res. 1999. PMID: 11094407 Free PMC article. Review. No abstract available.
-
Series introduction: the transcription factor NF-kappaB and human disease.J Clin Invest. 2001 Jan;107(1):3-6. doi: 10.1172/JCI11891. J Clin Invest. 2001. PMID: 11134170 Free PMC article. Review. No abstract available.
-
Curcumin mediated suppression of nuclear factor-κB promotes chondrogenic differentiation of mesenchymal stem cells in a high-density co-culture microenvironment.Arthritis Res Ther. 2010;12(4):R127. doi: 10.1186/ar3065. Epub 2010 Jul 1. Arthritis Res Ther. 2010. PMID: 20594343 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

