Impaired osteoclastic bone resorption leads to osteopetrosis in cathepsin-K-deficient mice
- PMID: 9811821
- PMCID: PMC24840
- DOI: 10.1073/pnas.95.23.13453
Impaired osteoclastic bone resorption leads to osteopetrosis in cathepsin-K-deficient mice
Abstract
Cathepsin K is a recently identified lysosomal cysteine proteinase. It is abundant in osteoclasts, where it is believed to play a vital role in the resorption and remodeling of bone. Pycnodysostosis is a rare inherited osteochondrodysplasia that is caused by mutations of the cathepsin-K gene, characterized by osteosclerosis, short stature, and acroosteolysis of the distal phalanges. With a view to delineating the role of cathepsin K in bone resorption, we generated mice with a targeted disruption of this proteinase. Cathepsin-K-deficient mice survive and are fertile, but display an osteopetrotic phenotype with excessive trabeculation of the bone-marrow space. Cathepsin-K-deficient osteoclasts manifested a modified ultrastructural appearance: their resorptive surface was poorly defined with a broad demineralized matrix fringe containing undigested fine collagen fibrils; their ruffled borders lacked crystal-like inclusions, and they were devoid of collagen-fibril-containing cytoplasmic vacuoles. Assaying the resorptive activity of cathepsin-K-deficient osteoclasts in vitro revealed this function to be severely impaired, which supports the contention that cathepsin K is of major importance in bone remodeling.
Figures
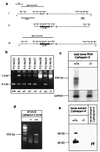
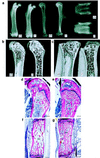
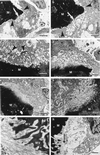
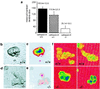
Similar articles
-
Functions of cathepsin K in bone resorption. Lessons from cathepsin K deficient mice.Adv Exp Med Biol. 2000;477:293-303. doi: 10.1007/0-306-46826-3_32. Adv Exp Med Biol. 2000. PMID: 10849757
-
The role of cathepsin K in normal bone resorption.Drug News Perspect. 2004 Jan-Feb;17(1):19-28. doi: 10.1358/dnp.2004.17.1.829022. Drug News Perspect. 2004. PMID: 14993931 Review.
-
Pycnodysostosis: role and regulation of cathepsin K in osteoclast function and human disease.Curr Mol Med. 2002 Aug;2(5):407-21. doi: 10.2174/1566524023362401. Curr Mol Med. 2002. PMID: 12125807 Review.
-
Cathepsin K knockout mice develop osteopetrosis due to a deficit in matrix degradation but not demineralization.J Bone Miner Res. 1999 Oct;14(10):1654-63. doi: 10.1359/jbmr.1999.14.10.1654. J Bone Miner Res. 1999. PMID: 10491212
-
Decreased bone turnover and deterioration of bone structure in two cases of pycnodysostosis.J Clin Endocrinol Metab. 2004 Apr;89(4):1538-47. doi: 10.1210/jc.2003-031055. J Clin Endocrinol Metab. 2004. PMID: 15070910 Review.
Cited by
-
Origins, Biology, and Diseases of Tissue Macrophages.Annu Rev Immunol. 2021 Apr 26;39:313-344. doi: 10.1146/annurev-immunol-093019-111748. Annu Rev Immunol. 2021. PMID: 33902313 Free PMC article. Review.
-
Odanacatib, a selective cathepsin K inhibitor to treat osteoporosis: safety, tolerability, pharmacokinetics and pharmacodynamics--results from single oral dose studies in healthy volunteers.Br J Clin Pharmacol. 2013 May;75(5):1240-54. doi: 10.1111/j.1365-2125.2012.04471.x. Br J Clin Pharmacol. 2013. PMID: 23013236 Free PMC article. Clinical Trial.
-
Cathepsin K: The Action in and Beyond Bone.Front Cell Dev Biol. 2020 Jun 4;8:433. doi: 10.3389/fcell.2020.00433. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32582709 Free PMC article. Review.
-
A mutation in CTSK gene in an autosomal recessive pycnodysostosis family of Pakistani origin.BMC Med Genet. 2009 Aug 12;10:76. doi: 10.1186/1471-2350-10-76. BMC Med Genet. 2009. PMID: 19674475 Free PMC article.
-
Neuronal loss and brain atrophy in mice lacking cathepsins B and L.Proc Natl Acad Sci U S A. 2002 Jun 11;99(12):7883-8. doi: 10.1073/pnas.112632299. Epub 2002 Jun 4. Proc Natl Acad Sci U S A. 2002. PMID: 12048238 Free PMC article.
References
-
- Erlebacher A, Filvaroff E H, Gitelman S E, Derynck R. Cell. 1995;80:371–378. - PubMed
-
- Delaisse J M, Eeckout Y, Vaes G. Biochem Biophys Res Commun. 1984;125:441–447. - PubMed
-
- Everts V, Delaisse J M, Korper W, Niehof A, Vaes G, Beertsen W. J Cell Physiol. 1992;150:221–231. - PubMed
-
- Hill P A, Buttle D J, Jones S J, Boyde A, Murata M, Reynolds J J, Meikle M C. J Cell Biochem. 1994;56:118–130. - PubMed
-
- Tezuka K, Tezuka Y, Maejima A, Sato T, Nemoto K, Kamioka H, Hakeda Y, Kumegawa M. J Biol Chem. 1994;269:1106–1109. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

