Altered xanthophyll compositions adversely affect chlorophyll accumulation and nonphotochemical quenching in Arabidopsis mutants
- PMID: 9789087
- PMCID: PMC23800
- DOI: 10.1073/pnas.95.22.13324
Altered xanthophyll compositions adversely affect chlorophyll accumulation and nonphotochemical quenching in Arabidopsis mutants
Abstract
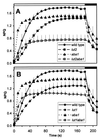
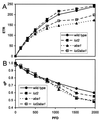
Collectively, the xanthophyll class of carotenoids perform a variety of critical roles in light harvesting antenna assembly and function. The xanthophyll composition of higher plant photosystems (lutein, violaxanthin, and neoxanthin) is remarkably conserved, suggesting important functional roles for each. We have taken a molecular genetic approach in Arabidopsis toward defining the respective roles of individual xanthophylls in vivo by using a series of mutant lines that selectively eliminate and substitute a range of xanthophylls. The mutations, lut1 and lut2 (lut = lutein deficient), disrupt lutein biosynthesis. In lut2, lutein is replaced mainly by a stoichiometric increase in violaxanthin and antheraxanthin. A third mutant, aba1, accumulates normal levels of lutein and substitutes zeaxanthin for violaxanthin and neoxanthin. The lut2aba1 double mutant completely lacks lutein, violaxanthin, and neoxanthin and instead accumulates zeaxanthin. All mutants were viable in soil and had chlorophyll a/b ratios ranging from 2.9 to 3.5 and near wild-type rates of photosynthesis. However, mutants accumulating zeaxanthin exhibited a delayed greening virescent phenotype, which was most severe and often lethal when zeaxanthin was the only xanthophyll present. Chlorophyll fluorescence quenching kinetics indicated that both zeaxanthin and lutein contribute to nonphotochemical quenching; specifically, lutein contributes, directly or indirectly, to the rapid rise of nonphotochemical quenching. The results suggest that the normal complement of xanthophylls, while not essential, is required for optimal assembly and function of the light harvesting antenna in higher plants.
Figures


Similar articles
-
Xanthophyll biosynthetic mutants of Arabidopsis thaliana: altered nonphotochemical quenching of chlorophyll fluorescence is due to changes in Photosystem II antenna size and stability.Biochim Biophys Acta. 2002 Feb 15;1553(3):309-19. doi: 10.1016/s0005-2728(02)00184-6. Biochim Biophys Acta. 2002. PMID: 11997140
-
Arabidopsis carotenoid mutants demonstrate that lutein is not essential for photosynthesis in higher plants.Plant Cell. 1996 Sep;8(9):1627-39. doi: 10.1105/tpc.8.9.1627. Plant Cell. 1996. PMID: 8837513 Free PMC article.
-
Photosynthesis, chlorophyll fluorescence, light-harvesting system and photoinhibition resistance of a zeaxanthin-accumulating mutant of Arabidopsis thaliana.J Photochem Photobiol B. 1996 Jun;34(1):87-94. doi: 10.1016/1011-1344(95)07272-1. J Photochem Photobiol B. 1996. PMID: 8765663
-
Genetic manipulation of carotenoid biosynthesis and photoprotection.Philos Trans R Soc Lond B Biol Sci. 2000 Oct 29;355(1402):1395-403. doi: 10.1098/rstb.2000.0701. Philos Trans R Soc Lond B Biol Sci. 2000. PMID: 11127994 Free PMC article. Review.
-
Molecular genetics of xanthophyll-dependent photoprotection in green algae and plants.Philos Trans R Soc Lond B Biol Sci. 2000 Oct 29;355(1402):1385-94. doi: 10.1098/rstb.2000.0700. Philos Trans R Soc Lond B Biol Sci. 2000. PMID: 11127993 Free PMC article. Review.
Cited by
-
Direct interaction of the major light-harvesting complex II and PsbS in nonphotochemical quenching.Proc Natl Acad Sci U S A. 2013 Apr 2;110(14):5452-6. doi: 10.1073/pnas.1205561110. Epub 2013 Mar 18. Proc Natl Acad Sci U S A. 2013. PMID: 23509270 Free PMC article.
-
An In Vivo Quantitative Comparison of Photoprotection in Arabidopsis Xanthophyll Mutants.Front Plant Sci. 2016 Jun 21;7:841. doi: 10.3389/fpls.2016.00841. eCollection 2016. Front Plant Sci. 2016. PMID: 27446097 Free PMC article.
-
Lutein is needed for efficient chlorophyll triplet quenching in the major LHCII antenna complex of higher plants and effective photoprotection in vivo under strong light.BMC Plant Biol. 2006 Dec 27;6:32. doi: 10.1186/1471-2229-6-32. BMC Plant Biol. 2006. PMID: 17192177 Free PMC article.
-
Transcriptional Regulation of Carotenoid Biosynthesis in Plants: So Many Regulators, So Little Consensus.Front Plant Sci. 2019 Aug 9;10:1017. doi: 10.3389/fpls.2019.01017. eCollection 2019. Front Plant Sci. 2019. PMID: 31447877 Free PMC article. Review.
-
A genome's-eye view of the light-harvesting polypeptides of Chlamydomonas reinhardtii.Curr Genet. 2004 Feb;45(2):61-75. doi: 10.1007/s00294-003-0460-x. Epub 2003 Dec 2. Curr Genet. 2004. PMID: 14652691 Review.
References
-
- Strain H. In: Biochemistry of Chloroplasts. Goodwin T, editor. I. New York: Academic; 1966. pp. 387–406.
-
- Grossman A R, Bhaya D, Apt K E, Kehoe D M. Annu Rev Genet. 1995;29:231–288. - PubMed
-
- Peter G F, Thornber J P. J Biol Chem. 1991;266:16745–16754. - PubMed
-
- Green B R, Durnford D G. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:685–714. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

