Guanine nucleotide exchange factor GEF115 specifically mediates activation of Rho and serum response factor by the G protein alpha subunit Galpha13
- PMID: 9789025
- PMCID: PMC23675
- DOI: 10.1073/pnas.95.22.12973
Guanine nucleotide exchange factor GEF115 specifically mediates activation of Rho and serum response factor by the G protein alpha subunit Galpha13
Abstract
Signal transduction pathways that mediate activation of serum response factor (SRF) by heterotrimeric G protein alpha subunits were characterized in transfection systems. Galphaq, Galpha12, and Galpha13, but not Galphai, activate SRF through RhoA. When Galphaq, alpha12, or alpha13 were coexpressed with a Rho-specific guanine nucleotide exchange factor GEF115, Galpha13, but not Galphaq or Galpha12, showed synergistic activation of SRF with GEF115. The synergy between Galpha13 and GEF115 depends on the N-terminal part of GEF115, and there was no synergistic effect between Galpha13 and another Rho-specific exchange factor Lbc. In addition, the Dbl-homology (DH)-domain-deletion mutant of GEF115 inhibited Galpha13- and Galpha12-induced, but not GEF115 itself- or Galphaq-induced, SRF activation. The DH-domain-deletion mutant also suppressed thrombin- and lysophosphatidic acid-induced SRF activation in NIH 3T3 cells, probably by inhibition of Galpha12/13. The N-terminal part of GEF115 contains a sequence motif that is homologous to the regulator of G protein signaling (RGS) domain of RGS12. RGS12 can inhibit both Galpha12 and Galpha13. Thus, the inhibition of Galpha12/13 by the DH-deletion mutant may be due to the RGS activity of the mutant. The synergism between Galpha13 and GEF115 indicates that GEF115 mediates Galpha13-induced activation of Rho and SRF.
Figures
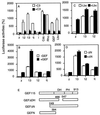
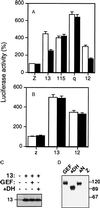
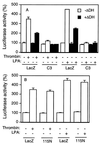

Similar articles
-
Specific involvement of G proteins in regulation of serum response factor-mediated gene transcription by different receptors.J Biol Chem. 1998 Oct 16;273(42):27118-23. doi: 10.1074/jbc.273.42.27118. J Biol Chem. 1998. PMID: 9765229
-
Tec/Bmx non-receptor tyrosine kinases are involved in regulation of Rho and serum response factor by Galpha12/13.EMBO J. 1998 Oct 1;17(19):5638-46. doi: 10.1093/emboj/17.19.5638. EMBO J. 1998. PMID: 9755164 Free PMC article.
-
Divergent C-terminal motifs in Gα12 and Gα13 provide distinct mechanisms of effector binding and SRF activation.Cell Signal. 2020 Aug;72:109653. doi: 10.1016/j.cellsig.2020.109653. Epub 2020 Apr 21. Cell Signal. 2020. PMID: 32330601
-
The GAPs, GEFs, and GDIs of heterotrimeric G-protein alpha subunits.Int J Biol Sci. 2005;1(2):51-66. doi: 10.7150/ijbs.1.51. Epub 2005 Apr 1. Int J Biol Sci. 2005. PMID: 15951850 Free PMC article. Review.
-
GPCR-Gα13 Involvement in Mitochondrial Function, Oxidative Stress, and Prostate Cancer.Int J Mol Sci. 2024 Jun 28;25(13):7162. doi: 10.3390/ijms25137162. Int J Mol Sci. 2024. PMID: 39000269 Free PMC article. Review.
Cited by
-
Modification of p115RhoGEF Ser(330) regulates its RhoGEF activity.Cell Signal. 2013 Nov;25(11):2085-92. doi: 10.1016/j.cellsig.2013.06.012. Epub 2013 Jun 29. Cell Signal. 2013. PMID: 23816534 Free PMC article.
-
Expression of Galpha 13 (Q226L) induces P19 stem cells to primitive endoderm via MEKK1, 2, or 4.J Biol Chem. 2002 Feb 1;277(5):3530-6. doi: 10.1074/jbc.M107031200. Epub 2001 Nov 7. J Biol Chem. 2002. PMID: 11700306 Free PMC article.
-
Signalling mechanisms of RhoGTPase regulation by the heterotrimeric G proteins G12 and G13.J Biochem. 2011 Oct;150(4):357-69. doi: 10.1093/jb/mvr105. Epub 2011 Aug 26. J Biochem. 2011. PMID: 21873336 Free PMC article. Review.
-
A Gα12-specific Binding Domain in AKAP-Lbc and p114RhoGEF.J Mol Signal. 2016 Sep 9;11:3. doi: 10.5334/1750-2187-11-3. J Mol Signal. 2016. PMID: 31051012 Free PMC article.
-
Profiling of differentially expressed genes using suppression subtractive hybridization in an equine model of chronic asthma.PLoS One. 2012;7(1):e29440. doi: 10.1371/journal.pone.0029440. Epub 2012 Jan 3. PLoS One. 2012. PMID: 22235296 Free PMC article.
References
-
- Simon M I, Strathman M P, Gautum M. Science. 1991;252:802–808. - PubMed
-
- Birnbaumer L, Birnbaumer M. J Recept Signal Transduct Res. 1995;15:213–252. - PubMed
-
- Gilman A G. Annu Rev Biochem. 1987;56:615–649. - PubMed
-
- Bourne H R. Curr Opin Cell Biol. 1997;9:134–142. - PubMed
-
- Hepler J R, Gilman A G. Trends Biochem Sci. 1992;17:383–387. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

