Host microvasculature influence on tumor vascular morphology and endothelial gene expression
- PMID: 9777955
- PMCID: PMC1853053
- DOI: 10.1016/S0002-9440(10)65668-4
Host microvasculature influence on tumor vascular morphology and endothelial gene expression
Abstract
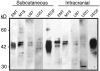
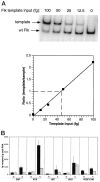
We have previously demonstrated that vascular endothelial growth factor-165 (VEGF), a tumor-secreted angiogenic factor, can acutely and chronically induce fenestrations in microvascular endothelium (Cancer Res 1997, 57:765-772). Because the morphology and function of microvascular endothelium differs from tissue to tissue, we undertook studies to examine whether the neovasculature in tumors also differed depending upon tumor location. Four tumor types implanted in the brain or subcutis in nude mice were studied: a murine rhabdomyosarcoma (M1S), a murine mammary carcinoma (EMT), and two human glioblastomas (U87 and U251). In addition, we studied Chinese hamster ovary cells stably transfected with human VEGF165. As previously reported, tumors grown in the subcutaneous space had a microvasculature that was fenestrated and had open endothelial gaps. The identical tumors when grown in the brain also had fenestrated endothelium and vessels with open endothelial gaps, but they were drastically reduced in occurrence. Open endothelial gaps were not seen in all tumors implanted in the brain (EMT and M1S), although fenestrated endothelium was always seen. VEGF and VEGF receptors were measured in tumors from both locations by immunoblotting and competitive polymerase chain reaction, respectively. VEGF amount was not significantly different between the tumor locations. Interestingly, total tumor vascular mRNA expression of both Flk-1 and Flt-1 was greater in tumor vessels derived from the brain compared with tumor vessels derived from subcutaneous tissues. These results demonstrate that the host microvascular environment determines the morphology and function of the tumor vasculature and that endothelia from different tissues vary in their ability to express the VEGF receptors given identical stimuli.
Figures

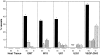
Similar articles
-
Neovasculature induced by vascular endothelial growth factor is fenestrated.Cancer Res. 1997 Feb 15;57(4):765-72. Cancer Res. 1997. PMID: 9044858
-
Expression of vascular endothelial growth factor and its receptors in the anaplastic progression of astrocytoma, oligodendroglioma, and ependymoma.Am J Surg Pathol. 1998 Jul;22(7):816-26. doi: 10.1097/00000478-199807000-00004. Am J Surg Pathol. 1998. PMID: 9669344
-
Transforming growth factor-beta and Ras regulate the VEGF/VEGF-receptor system during tumor angiogenesis.Int J Cancer. 2002 Jan 10;97(2):142-8. doi: 10.1002/ijc.1599. Int J Cancer. 2002. PMID: 11774256
-
Possible involvement of VEGF-FLT tyrosine kinase receptor system in normal and tumor angiogenesis.Princess Takamatsu Symp. 1994;24:162-70. Princess Takamatsu Symp. 1994. PMID: 8983073 Review.
-
The splice variants of vascular endothelial growth factor (VEGF) and their receptors.J Cell Sci. 2001 Mar;114(Pt 5):853-65. doi: 10.1242/jcs.114.5.853. J Cell Sci. 2001. PMID: 11181169 Review.
Cited by
-
Hypoxia, notch signalling, and prostate cancer.Nat Rev Urol. 2013 Jul;10(7):405-13. doi: 10.1038/nrurol.2013.110. Epub 2013 May 28. Nat Rev Urol. 2013. PMID: 23712204 Free PMC article. Review.
-
Octreotide-Targeted Lcn2 siRNA PEGylated Liposomes as a Treatment for Metastatic Breast Cancer.Bioengineering (Basel). 2021 Apr 3;8(4):44. doi: 10.3390/bioengineering8040044. Bioengineering (Basel). 2021. PMID: 33916786 Free PMC article.
-
Targeted delivery of nano-therapeutics for major disorders of the central nervous system.Pharm Res. 2013 Oct;30(10):2485-98. doi: 10.1007/s11095-013-1122-4. Pharm Res. 2013. PMID: 23797465 Review.
-
The role of PLVAP in endothelial cells.Cell Tissue Res. 2023 May;392(2):393-412. doi: 10.1007/s00441-023-03741-1. Epub 2023 Feb 13. Cell Tissue Res. 2023. PMID: 36781482 Free PMC article. Review.
-
Angiogenesis and Lymphangiogenesis in Medulloblastoma Development.Biology (Basel). 2023 Jul 21;12(7):1028. doi: 10.3390/biology12071028. Biology (Basel). 2023. PMID: 37508458 Free PMC article. Review.
References
-
- Liotta LA, Steeg PS, Stetler-Stevenson WG: Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell 1991, 64:327-336 - PubMed
-
- Ellis LM, Fidler IJ: Angiogenesis and metastasis. Eur J Cancer 1996, 32A:2451-2460 - PubMed
-
- Ferrara N, Davis-Smyth T: The biology of vascular endothelial growth factor. Endocr Rev 1997, 18:4-25 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

