Metabolic labeling of woodchuck hepatitis B virus X protein in naturally infected hepatocytes reveals a bimodal half-life and association with the nuclear framework
- PMID: 9765489
- PMCID: PMC110361
- DOI: 10.1128/JVI.72.11.9359-9364.1998
Metabolic labeling of woodchuck hepatitis B virus X protein in naturally infected hepatocytes reveals a bimodal half-life and association with the nuclear framework
Abstract
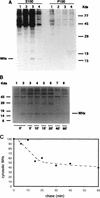
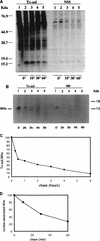
In order to identify potential sites of hepadnavirus X protein action, we have investigated the subcellular distribution and the stability of woodchuck hepatitis virus (WHV) X protein (WHx) in primary hepatocytes isolated from woodchucks with persistent WHV infection. In vivo cell labeling and cell fractionation studies showed that the majority of WHx is a soluble cytoplasmic protein while a minor part of newly synthesized WHx is associated with a nuclear framework fraction (20%) and with cytoskeletal components (5 to 10%). Pulse-chase experiments revealed that cytoplasmic WHx has a short half-life and decays with bimodal kinetics (approximately 20 min and 3 h). The rates of association and turnover of nucleus-associated WHx suggest that compartmentalization may be responsible for the bimodal turnover observed in the cytoplasm.
Figures


Similar articles
-
Woodchuck hepatitis virus X protein is present in chronically infected woodchuck liver and woodchuck hepatocellular carcinomas which are permissive for viral replication.J Virol. 1996 Aug;70(8):5246-54. doi: 10.1128/JVI.70.8.5246-5254.1996. J Virol. 1996. PMID: 8764034 Free PMC article.
-
Hepatic expression of the woodchuck hepatitis virus X-antigen during acute and chronic infection and detection of a woodchuck hepatitis virus X-antigen antibody response.Hepatology. 1997 Dec;26(6):1607-15. doi: 10.1002/hep.510260632. Hepatology. 1997. PMID: 9398005
-
Replication of naturally occurring woodchuck hepatitis virus deletion mutants in primary hepatocyte cultures and after transmission to naive woodchucks.J Virol. 2001 Apr;75(8):3811-8. doi: 10.1128/JVI.75.8.3811-3818.2001. J Virol. 2001. PMID: 11264370 Free PMC article.
-
The woodchuck model of hepatitis B virus infection.ILAR J. 2001;42(2):89-102. doi: 10.1093/ilar.42.2.89. ILAR J. 2001. PMID: 11406711 Review.
-
Occult persistence and lymphotropism of hepadnaviral infection: insights from the woodchuck viral hepatitis model.Immunol Rev. 2000 Apr;174:98-111. doi: 10.1034/j.1600-0528.2002.017406.x. Immunol Rev. 2000. PMID: 10807510 Review.
Cited by
-
Hepatitis B virus X protein stimulates viral genome replication via a DDB1-dependent pathway distinct from that leading to cell death.J Virol. 2005 Apr;79(7):4238-45. doi: 10.1128/JVI.79.7.4238-4245.2005. J Virol. 2005. PMID: 15767425 Free PMC article.
-
Differential effects on apoptosis induction in hepatocyte lines by stable expression of hepatitis B virus X protein.World J Gastroenterol. 2006 Aug 7;12(29):4673-82. doi: 10.3748/wjg.v12.i29.4673. World J Gastroenterol. 2006. PMID: 16937438 Free PMC article.
-
Hepatitis B virus HBx protein localized to the nucleus restores HBx-deficient virus replication in HepG2 cells and in vivo in hydrodynamically-injected mice.Virology. 2009 Jul 20;390(1):122-9. doi: 10.1016/j.virol.2009.05.001. Epub 2009 May 23. Virology. 2009. PMID: 19464721 Free PMC article.
-
Role of HBx in hepatitis B virus persistence and its therapeutic implications.Curr Opin Virol. 2018 Jun;30:32-38. doi: 10.1016/j.coviro.2018.01.007. Epub 2018 Feb 20. Curr Opin Virol. 2018. PMID: 29454995 Free PMC article. Review.
-
Turnover of hepatitis B virus X protein is regulated by damaged DNA-binding complex.J Virol. 2002 Jul;76(13):6495-501. doi: 10.1128/jvi.76.13.6495-6501.2002. J Virol. 2002. PMID: 12050362 Free PMC article.
References
-
- Aldrich C E, Coates L, Wu T T, Newbold J, Tennant B C, Summers J, Seeger C, Mason W S. In vitro infection of woodchuck hepatocytes with woodchuck hepatitis virus and ground squirrel hepatitis virus. Virology. 1989;172:247–252. - PubMed
-
- Amellem O, Stokke T, Sandvik J A, Pettersen E O. The retinoblastoma gene product is reversibly dephosphorylated and bound in the nucleus in S and G2 phases during hypoxic stress. Exp Cell Res. 1996;227:106–115. - PubMed
-
- Buendia M A. Hepatitis B viruses and hepatocellular carcinoma. Adv Cancer Res. 1992;59:167–226. - PubMed
-
- Capco D G, Wan K M, Penman S. The nuclear matrix: three-dimensional architecture and protein composition. Cell. 1982;29:847–858. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

