Walleye retroviruses associated with skin tumors and hyperplasias encode cyclin D homologs
- PMID: 9765420
- PMCID: PMC110292
- DOI: 10.1128/JVI.72.11.8765-8771.1998
Walleye retroviruses associated with skin tumors and hyperplasias encode cyclin D homologs
Abstract
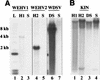
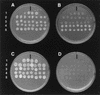
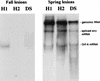
Walleye dermal sarcoma (WDS) and walleye epidermal hyperplasia (WEH) are skin diseases of walleye fish that appear and regress on a seasonal basis. We report here that the complex retroviruses etiologically associated with WDS (WDS virus [WDSV]) and WEH (WEH viruses 1 and 2 [WEHV1 and WEHV2, respectively]) encode D-type cyclin homologs. The retroviral cyclins (rv-cyclins) are distantly related to one another and to known cyclins and are not closely related to any walleye cellular gene based on low-stringency Southern blotting. Since aberrant expression of D-type cyclins occurs in many human tumors, we suggest that expression of the rv-cyclins may contribute to the development of WDS or WEH. In support of this hypothesis, we show that rv-cyclin transcripts are made in developing WDS and WEH and that the rv-cyclin of WDSV induces cell cycle progression in yeast (Saccharomyces cerevisiae). WEHV1, WEHV2, and WDSV are the first examples of retroviruses that encode cyclin homologs. WEH and WDS and their associated retroviruses represent a novel paradigm of retroviral tumor induction and, importantly, tumor regression.
Figures

Similar articles
-
Sequence and transcriptional analyses of the fish retroviruses walleye epidermal hyperplasia virus types 1 and 2: evidence for a gene duplication.J Virol. 1999 Nov;73(11):9393-403. doi: 10.1128/JVI.73.11.9393-9403.1999. J Virol. 1999. PMID: 10516048 Free PMC article.
-
Two closely related but distinct retroviruses are associated with walleye discrete epidermal hyperplasia.J Virol. 1998 Apr;72(4):3484-90. doi: 10.1128/JVI.72.4.3484-3490.1998. J Virol. 1998. PMID: 9525688 Free PMC article.
-
Squamous epithelial proliferation induced by walleye dermal sarcoma retrovirus cyclin in transgenic mice.Proc Natl Acad Sci U S A. 2000 May 23;97(11):6114-9. doi: 10.1073/pnas.110024497. Proc Natl Acad Sci U S A. 2000. PMID: 10811912 Free PMC article.
-
Pathology of tumors in fish associated with retroviruses: a review.Vet Pathol. 2013 May;50(3):390-403. doi: 10.1177/0300985813480529. Epub 2013 Mar 1. Vet Pathol. 2013. PMID: 23456970 Review.
-
Converging strategies in expression of human complex retroviruses.Viruses. 2011 Aug;3(8):1395-414. doi: 10.3390/v3081395. Epub 2011 Aug 11. Viruses. 2011. PMID: 21994786 Free PMC article. Review.
Cited by
-
Transgenic expression of walleye dermal sarcoma virus rv-cyclin (orfA) in zebrafish does not result in tissue proliferation.Mar Biotechnol (NY). 2011 Apr;13(2):142-50. doi: 10.1007/s10126-010-9274-2. Epub 2010 Mar 28. Mar Biotechnol (NY). 2011. PMID: 20349325 Free PMC article.
-
Characterization of the protease of a fish retrovirus, walleye dermal sarcoma virus.J Virol. 2002 May;76(9):4341-9. doi: 10.1128/jvi.76.9.4341-4349.2002. J Virol. 2002. PMID: 11932400 Free PMC article.
-
Walleye dermal sarcoma virus rv-cyclin inhibits NF-kappaB-dependent transcription.Virology. 2009 Mar 30;386(1):55-60. doi: 10.1016/j.virol.2008.12.026. Epub 2009 Jan 26. Virology. 2009. PMID: 19176230 Free PMC article.
-
Exploitation of the Mediator complex by viruses.PLoS Pathog. 2022 Apr 21;18(4):e1010422. doi: 10.1371/journal.ppat.1010422. eCollection 2022 Apr. PLoS Pathog. 2022. PMID: 35446926 Free PMC article. No abstract available.
-
The evolution, distribution and diversity of endogenous retroviruses.Virus Genes. 2003 May;26(3):291-315. doi: 10.1023/a:1024455415443. Virus Genes. 2003. PMID: 12876457 Review.
References
-
- Anders K, Yoshimizu M. Role of viruses in the induction of skin tumours and tumour-like proliferations of fish. Dis Aquat Org. 1994;19:215–232.
-
- Arnold A. The cyclin D1-PRAD1 oncogene in human neoplasia. J Investig Med. 1995;43:543–549. - PubMed
-
- Ausubel F M, Brent B, Kingston R E, Moore D D, Smith J A, Seidman J G, Struhl K. Current protocols in molecular biology. New York, N.Y: John Wiley and Sons; 1987.
-
- Bazan J F. Helical fold prediction for the cyclin box. Proteins Struct Funct Genet. 1996;24:1–17. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

