Senescence of human fibroblasts induced by oncogenic Raf
- PMID: 9765202
- PMCID: PMC317194
- DOI: 10.1101/gad.12.19.2997
Senescence of human fibroblasts induced by oncogenic Raf
Abstract
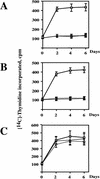
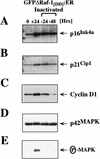
The oncogenes RAS and RAF came to view as agents of neoplastic transformation. However, in normal cells, these genes can have effects that run counter to oncogenic transformation, such as arrest of the cell division cycle, induction of cell differentiation, and apoptosis. Recent work has demonstrated that RAS elicits proliferative arrest and senescence in normal mouse and human fibroblasts. Because the Raf/MEK/MAP kinase signaling cascade is a key effector of signaling from Ras proteins, we examined the ability of conditionally active forms of Raf-1 to elicit cell cycle arrest and senescence in human cells. Activation of Raf-1 in nonimmortalized human lung fibroblasts (IMR-90) led to the prompt and irreversible arrest of cellular proliferation and the premature onset of senescence. Concomitant with the onset of cell cycle arrest, we observed the induction of the cyclin-dependent kinase (CDK) inhibitors p21(Cip1) and p16(Ink4a). Ablation of p53 and p21(Cip1) expression by use of the E6 oncoprotein of HPV16 demonstrated that expression of these proteins was not required for Raf-induced cell cycle arrest or senescence. Furthermore, cell cycle arrest and senescence were elicited in IMR-90 cells by the ectopic expression of p16(Ink4a) alone. Pharmacological inhibition of the Raf/MEK/MAP kinase cascade prevented Raf from inducing p16(Ink4a) and also prevented Raf-induced senescence. We conclude that the kinase cascade initiated by Raf can regulate the expression of p16(Ink4a) and the proliferative arrest and senescence that follows. Induction of senescence may provide a defense against neoplastic transformation when the MAP kinase signaling cascade is inappropriately active.
Figures






Similar articles
-
Dual growth arrest pathways in astrocytes and astrocytic tumors in response to Raf-1 activation.J Biol Chem. 2001 Jun 1;276(22):18871-7. doi: 10.1074/jbc.M011514200. Epub 2001 Mar 15. J Biol Chem. 2001. PMID: 11278920
-
Long-term growth arrest of PUVA-treated fibroblasts in G2/M in the absence of p16(INK4a) p21(CIP1) or p53.Exp Dermatol. 2003 Oct;12(5):629-37. doi: 10.1034/j.1600-0625.2003.00024.x. Exp Dermatol. 2003. PMID: 14705804
-
Depletion of ERK2 but not ERK1 abrogates oncogenic Ras-induced senescence.Cell Signal. 2013 Dec;25(12):2540-7. doi: 10.1016/j.cellsig.2013.08.014. Epub 2013 Aug 30. Cell Signal. 2013. PMID: 23993963
-
Tumor suppressors and oncogenes in cellular senescence.Exp Gerontol. 2000 May;35(3):317-29. doi: 10.1016/s0531-5565(00)00083-8. Exp Gerontol. 2000. PMID: 10832053 Review.
-
[Cyclin dependent kinase inhibitors and replicative senescence].Pathol Biol (Paris). 2001 Dec;49(10):830-9. doi: 10.1016/s0369-8114(01)00249-8. Pathol Biol (Paris). 2001. PMID: 11776695 Review. French.
Cited by
-
Gerosuppression in confluent cells.Aging (Albany NY). 2014 Dec;6(12):1010-8. doi: 10.18632/aging.100714. Aging (Albany NY). 2014. PMID: 25585637 Free PMC article.
-
Mammalian cells acquire epigenetic hallmarks of human cancer during immortalization.Nucleic Acids Res. 2013 Jan 7;41(1):182-95. doi: 10.1093/nar/gks1051. Epub 2012 Nov 11. Nucleic Acids Res. 2013. PMID: 23143272 Free PMC article.
-
A posttranslational modification cascade involving p38, Tip60, and PRAK mediates oncogene-induced senescence.Mol Cell. 2013 Jun 6;50(5):699-710. doi: 10.1016/j.molcel.2013.04.013. Epub 2013 May 16. Mol Cell. 2013. PMID: 23685072 Free PMC article.
-
The homeobox transcription factor HB9 induces senescence and blocks differentiation in hematopoietic stem and progenitor cells.Haematologica. 2019 Jan;104(1):35-46. doi: 10.3324/haematol.2018.189407. Epub 2018 Aug 9. Haematologica. 2019. PMID: 30093397 Free PMC article.
-
Cellular senescence is an important mechanism of tumor regression upon c-Myc inactivation.Proc Natl Acad Sci U S A. 2007 Aug 7;104(32):13028-33. doi: 10.1073/pnas.0701953104. Epub 2007 Jul 30. Proc Natl Acad Sci U S A. 2007. PMID: 17664422 Free PMC article.
References
-
- Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. - PubMed
-
- Avruch J, Zhang XF, Kyriakis JM. Raf meets Ras: Completing the framework of a signal transduction pathway. Trends Biochem Sci. 1994;19:279–283. - PubMed
-
- Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–352. - PubMed
-
- Brown JP, Wei W, Sedivy JM. Bypass of senescence after disruption of p21CIP1/WAF1 gene in normal diploid human fibroblasts. Science. 1997;277:831–834. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
