Sorting mechanisms regulating membrane protein traffic in the apical transcytotic pathway of polarized MDCK cells
- PMID: 9763422
- PMCID: PMC2132803
- DOI: 10.1083/jcb.143.1.81
Sorting mechanisms regulating membrane protein traffic in the apical transcytotic pathway of polarized MDCK cells
Abstract
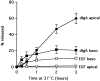
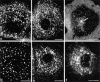
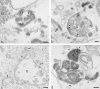
The transcytotic pathway followed by the polymeric IgA receptor (pIgR) carrying its bound ligand (dIgA) from the basolateral to the apical surface of polarized MDCK cells has been mapped using morphological tracers. At 20 degreesC dIgA-pIgR internalize to interconnected groups of vacuoles and tubules that comprise the endosomal compartment and in which they codistribute with internalized transferrin receptors (TR) and epidermal growth factor receptors (EGFR). Upon transfer to 37 degreesC the endosome vacuoles develop long tubules that give rise to a distinctive population of 100-nm-diam cup-shaped vesicles containing pIgR. At the same time, the endosome gives rise to multivesicular endosomes (MVB) enriched in EGFR and to 60-nm-diam basolateral vesicles. The cup-shaped vesicles carry the dIgA/pIgR complexes to the apical surface where they exocytose. Using video microscopy and correlative electron microscopy to study cells grown thin and flat we show that endosome vacuoles tubulate in response to dIgA/pIgR but that the tubules contain TR as well as pIgR. However, we show that TR are removed from these dIgA-induced tubules via clathrin-coated buds and, as a result, the cup-shaped vesicles to which the tubules give rise become enriched in dIgA/pIgR. Taken together with the published information available on pIgR trafficking signals, our observations suggest that the steady-state concentrations of TR and unoccupied pIgR on the basolateral surface of polarized MDCK cells are maintained by a signal-dependent, clathrin-based sorting mechanism that operates along the length of the transcytotic pathway. We propose that the differential sorting of occupied receptors within the MDCK endosome is achieved by this clathrin-based mechanism continuously retrieving receptors like TR from the pathways that deliver pIgR to the apical surface and EGFR to the lysosome.
Figures

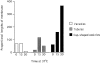

Similar articles
-
Apical and basolateral endosomes of MDCK cells are interconnected and contain a polarized sorting mechanism.J Cell Biol. 1996 Oct;135(1):139-52. doi: 10.1083/jcb.135.1.139. J Cell Biol. 1996. PMID: 8858169 Free PMC article.
-
In polarized MDCK cells basolateral vesicles arise from clathrin-gamma-adaptin-coated domains on endosomal tubules.J Cell Biol. 1998 May 4;141(3):611-23. doi: 10.1083/jcb.141.3.611. J Cell Biol. 1998. PMID: 9566963 Free PMC article.
-
Basolateral to apical transcytosis in polarized cells is indirect and involves BFA and trimeric G protein sensitive passage through the apical endosome.J Cell Biol. 1994 Jan;124(1-2):83-100. doi: 10.1083/jcb.124.1.83. J Cell Biol. 1994. PMID: 7905002 Free PMC article.
-
Regulation of protein traffic in polarized epithelial cells.Histol Histopathol. 1995 Apr;10(2):423-31. Histol Histopathol. 1995. PMID: 7599439 Review.
-
Molecular sorting in polarized and non-polarized cells: common problems, common solutions.J Cell Sci Suppl. 1993;17:1-7. doi: 10.1242/jcs.1993.supplement_17.1. J Cell Sci Suppl. 1993. PMID: 8144683 Review.
Cited by
-
Apical protein transport and lumen morphogenesis in polarized epithelial cells.Biosci Rep. 2011 Aug;31(4):245-56. doi: 10.1042/BSR20100119. Biosci Rep. 2011. PMID: 21366541 Free PMC article. Review.
-
Cell models for studying renal physiology.Pflugers Arch. 2008 Oct;457(1):1-15. doi: 10.1007/s00424-008-0507-4. Epub 2008 Apr 22. Pflugers Arch. 2008. PMID: 18427833 Review.
-
Bidirectional transepithelial IgG transport by a strongly polarized basolateral membrane Fcgamma-receptor.Mol Biol Cell. 2004 Apr;15(4):1746-59. doi: 10.1091/mbc.e03-11-0832. Epub 2004 Feb 6. Mol Biol Cell. 2004. PMID: 14767057 Free PMC article.
-
Differential trafficking of transforming growth factor-beta receptors and ligand in polarized epithelial cells.Mol Biol Cell. 2004 Jun;15(6):2853-62. doi: 10.1091/mbc.e04-02-0097. Epub 2004 Apr 9. Mol Biol Cell. 2004. PMID: 15075369 Free PMC article.
-
EEA1, a tethering protein of the early sorting endosome, shows a polarized distribution in hippocampal neurons, epithelial cells, and fibroblasts.Mol Biol Cell. 2000 Aug;11(8):2657-71. doi: 10.1091/mbc.11.8.2657. Mol Biol Cell. 2000. PMID: 10930461 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

