Isolation of yeast mutants defective for localization of vacuolar vital dyes
- PMID: 9751732
- PMCID: PMC21707
- DOI: 10.1073/pnas.95.20.11721
Isolation of yeast mutants defective for localization of vacuolar vital dyes
Abstract
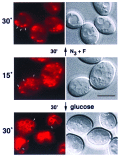
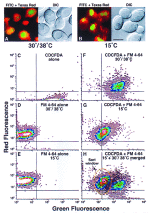
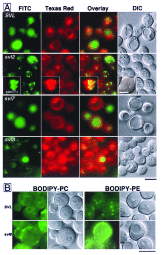
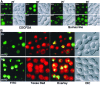
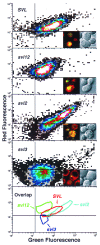
An application of flow cytometric sorting is used for isolation of Saccharomyces cerevisiae mutants that mislocalize vacuolar vital dyes. This screen is based on the ability of a lipophilic styryl compound, N-(3-triethylammoniumpropyl)-4-(6-(4-(diethylamino)phenyl)hexatrie nyl )pyridinium dibromide (FM4-64), to label endocytic intermediates from the plasma membrane to the vacuole membrane at 15 degreesC. Cells stained at 15 degreesC for both FM4-64 and carboxydichlorofluorescein diacetate (a vacuolar luminal vital stain), had a pronounced shift in red/green fluorescence from cells stained at 30 degrees or 38 degreesC. Flow cytometric selection based on this characteristic shift allowed the isolation of 16 mutants. These comprised 12 complementation groups, which we have designated SVL for styryl dye vacuolar localization. These groups were put into three classes. Class I mutants contain very large vacuoles; class II mutants have very fragmented vacuoles; and class III mutants show the strongest svl phenotype with punctate/diffuse FM4-64 staining. Limited genetic overlap was observed with previously isolated mutants, namely svl2/vps41, svl6/vps16, and svl7/fab1. The remaining svl mutants appear to represent novel genes, two of which showed temperature-sensitive vacuole staining morphology. Another mutant, svl8, displayed defects in uptake and sorting of phosphatidylcholine and phosphatidylethanolamine. Our flow cytometric strategy may be useful for isolation of other mutants where mislocalization of fluorescent compounds can be detected.
Figures
Similar articles
-
A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast.J Cell Biol. 1995 Mar;128(5):779-92. doi: 10.1083/jcb.128.5.779. J Cell Biol. 1995. PMID: 7533169 Free PMC article.
-
A novel fluorescence-activated cell sorter-based screen for yeast endocytosis mutants identifies a yeast homologue of mammalian eps15.J Cell Biol. 1996 Dec;135(6 Pt 1):1485-500. doi: 10.1083/jcb.135.6.1485. J Cell Biol. 1996. PMID: 8978817 Free PMC article.
-
Endocytosis and vacuolar morphology in Saccharomyces cerevisiae are altered in response to ethanol stress or heat shock.Yeast. 1999 Sep 15;15(12):1211-22. doi: 10.1002/(SICI)1097-0061(19990915)15:12<1211::AID-YEA448>3.0.CO;2-H. Yeast. 1999. PMID: 10487923
-
FM-dyes as experimental probes for dissecting vesicle trafficking in living plant cells.J Microsc. 2004 May;214(Pt 2):159-73. doi: 10.1111/j.0022-2720.2004.01348.x. J Microsc. 2004. PMID: 15102063 Review.
-
Flow cytometry/cell sorting for isolating membrane trafficking mutants in yeast.Methods Enzymol. 2002;351:623-31. doi: 10.1016/s0076-6879(02)51872-3. Methods Enzymol. 2002. PMID: 12073372 Review. No abstract available.
Cited by
-
Synthesis and function of membrane phosphoinositides in budding yeast, Saccharomyces cerevisiae.Biochim Biophys Acta. 2007 Mar;1771(3):353-404. doi: 10.1016/j.bbalip.2007.01.015. Epub 2007 Feb 6. Biochim Biophys Acta. 2007. PMID: 17382260 Free PMC article. Review.
-
Candida albicans ENT2 Contributes to Efficient Endocytosis, Cell Wall Integrity, Filamentation, and Virulence.mSphere. 2021 Oct 27;6(5):e0070721. doi: 10.1128/mSphere.00707-21. Epub 2021 Sep 29. mSphere. 2021. PMID: 34585966 Free PMC article.
-
Gene Amplification on Demand Accelerates Cellobiose Utilization in Engineered Saccharomyces cerevisiae.Appl Environ Microbiol. 2016 May 31;82(12):3631-3639. doi: 10.1128/AEM.00410-16. Print 2016 Jun 15. Appl Environ Microbiol. 2016. PMID: 27084006 Free PMC article.
-
Mutations that affect vacuole biogenesis inhibit proliferation of the endoplasmic reticulum in Saccharomyces cerevisiae.Genetics. 2002 Apr;160(4):1335-52. doi: 10.1093/genetics/160.4.1335. Genetics. 2002. PMID: 11973291 Free PMC article.
-
In vivo role for actin-regulating kinases in endocytosis and yeast epsin phosphorylation.Mol Biol Cell. 2001 Nov;12(11):3668-79. doi: 10.1091/mbc.12.11.3668. Mol Biol Cell. 2001. PMID: 11694597 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

