Blocking cytochrome c activity within intact neurons inhibits apoptosis
- PMID: 9744886
- PMCID: PMC2141762
- DOI: 10.1083/jcb.142.6.1583
Blocking cytochrome c activity within intact neurons inhibits apoptosis
Abstract
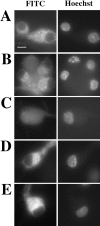
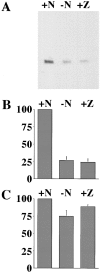
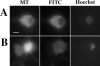
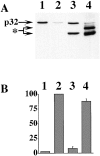
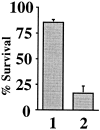
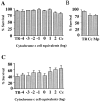
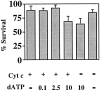
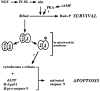
Cytochrome c has been shown to play a role in cell-free models of apoptosis. During NGF withdrawal-induced apoptosis of intact rat superior cervical ganglion (SCG) neurons, we observe the redistribution of cytochrome c from the mitochondria to the cytoplasm. This redistribution is not inhibited by the caspase inhibitor Z-Val-Ala-Asp-fluoromethylketone (ZVADfmk) but is blocked by either of the neuronal survival agents 8-(4-chlorophenylthio)adenosine 3':5'-cyclic monophosphate (CPT-cAMP) or cycloheximide. Moreover, microinjection of SCG neurons with antibody to cytochrome c blocks NGF withdrawal-induced apoptosis. However, microinjection of SCG neurons with cytochrome c does not alter the rate of apoptosis in either the presence or absence of NGF. These data suggest that cytochrome c is an intrinsic but not limiting component of the neuronal apoptotic pathway.
Figures

Similar articles
-
Evidence for redox regulation of cytochrome C release during programmed neuronal death: antioxidant effects of protein synthesis and caspase inhibition.J Neurosci. 2001 Mar 15;21(6):1949-63. doi: 10.1523/JNEUROSCI.21-06-01949.2001. J Neurosci. 2001. PMID: 11245680 Free PMC article.
-
Evidence of a novel event during neuronal death: development of competence-to-die in response to cytoplasmic cytochrome c.Neuron. 1998 Oct;21(4):695-705. doi: 10.1016/s0896-6273(00)80587-5. Neuron. 1998. PMID: 9808457
-
Neurotrophic factors prevent ceramide-induced apoptosis downstream of c-Jun N-terminal kinase activation in PC12 cells.J Neurochem. 1998 Jul;71(1):161-9. doi: 10.1046/j.1471-4159.1998.71010161.x. J Neurochem. 1998. PMID: 9648862
-
The release of cytochrome c from mitochondria during apoptosis of NGF-deprived sympathetic neurons is a reversible event.J Cell Biol. 1999 Mar 8;144(5):883-9. doi: 10.1083/jcb.144.5.883. J Cell Biol. 1999. PMID: 10085288 Free PMC article.
-
Phosphatidylinositol 3-kinase is required for the trophic, but not the survival-promoting, actions of NGF on sympathetic neurons.J Neurosci. 2000 Oct 1;20(19):7228-37. doi: 10.1523/JNEUROSCI.20-19-07228.2000. J Neurosci. 2000. PMID: 11007879 Free PMC article.
Cited by
-
Inhibition of NGF deprivation-induced death by low oxygen involves suppression of BIMEL and activation of HIF-1.J Cell Biol. 2005 Mar 14;168(6):911-20. doi: 10.1083/jcb.200407079. J Cell Biol. 2005. PMID: 15767462 Free PMC article.
-
Decreased apoptosome activity with neuronal differentiation sets the threshold for strict IAP regulation of apoptosis.J Cell Biol. 2004 Oct 25;167(2):303-13. doi: 10.1083/jcb.200406073. J Cell Biol. 2004. PMID: 15504912 Free PMC article.
-
Cytochrome c is released in a reactive oxygen species-dependent manner and is degraded via caspase-like proteases in tobacco Bright-Yellow 2 cells en route to heat shock-induced cell death.Plant Physiol. 2006 May;141(1):208-19. doi: 10.1104/pp.106.078683. Epub 2006 Mar 10. Plant Physiol. 2006. PMID: 16531480 Free PMC article.
-
Evidence for redox regulation of cytochrome C release during programmed neuronal death: antioxidant effects of protein synthesis and caspase inhibition.J Neurosci. 2001 Mar 15;21(6):1949-63. doi: 10.1523/JNEUROSCI.21-06-01949.2001. J Neurosci. 2001. PMID: 11245680 Free PMC article.
-
Caspase inhibition extends the commitment to neuronal death beyond cytochrome c release to the point of mitochondrial depolarization.J Cell Biol. 2000 Jul 10;150(1):131-43. doi: 10.1083/jcb.150.1.131. J Cell Biol. 2000. PMID: 10893262 Free PMC article.
References
-
- Antonsson B, Conti F, Ciavatta A, Montessuit S, Lewis S, Martinou I, Bernasconi L, Bernard A, Mermod J-J, Mazzei G, et al. Inhibition of Bax channel-forming activity by Bcl-2. Science. 1997;277:370–372. - PubMed
-
- Bortner CD, Oldenburg NBE, Cidlowski JA. The role of DNA fragmentation in apoptosis. Trends Cell Biol. 1995;5:21–26. - PubMed
-
- Bredesen DE. Neural apoptosis. Ann Neurol. 1995;38:839–851. - PubMed
-
- Brischwein K, Engelcke M, Riedinger HJ, Probst H. Role of ribonucleotide reductase and deoxynucleotide pools in the oxygen-dependent control of DNA replication in Ehrlich ascites cells. Eur J Biochem. 1997;244:286–293. - PubMed

