Point mutations in the WD40 domain of Eed block its interaction with Ezh2
- PMID: 9742080
- PMCID: PMC109149
- DOI: 10.1128/MCB.18.10.5634
Point mutations in the WD40 domain of Eed block its interaction with Ezh2
Abstract
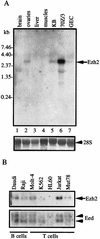
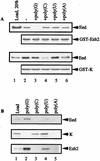
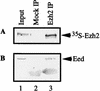
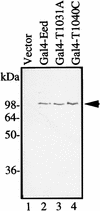
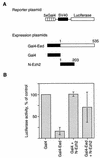
The Polycomb group proteins are involved in maintenance of the silenced state of several developmentally regulated genes. These proteins form large aggregates with different subunit compositions. To explore the nature of these complexes and their function, we used the full-length Eed (embryonic ectoderm development) protein, a mammalian homolog of the Drosophila Polycomb group protein Esc, as a bait in the yeast two-hybrid screen. Several strongly interacting cDNA clones were isolated. The cloned cDNAs all encoded the 150- to 200-amino-acid N-terminal fragment of the mammalian homolog of the Drosophila Enhancer of zeste [E(z)] protein, Ezh2. The full-length Ezh2 bound strongly to Eed in vitro, and Eed coimmunoprecipitated with Ezh2 from murine 70Z/3 cell extracts, confirming the interaction between these proteins observed in yeast. Mutations T1031A and T1040C in one of the WD40 repeats of Eed, which account for the hypomorphic and lethal phenotype of eed in mouse development, blocked binding of Ezh2 to Eed in a two-hybrid interaction in yeast and in mammalian cells. These mutations also blocked the interaction between these proteins in vitro. In mammalian cells, the Gal4-Eed fusion protein represses the activity of a promoter bearing Gal4 DNA elements. The N-terminal fragment of the Ezh2 protein abolished the transcriptional repressor activity of Gal4-Eed protein when they were coexpressed in mammalian cells. Eed and Ezh2 were also found to bind RNA in vitro, and RNA altered the interaction between these proteins. These findings suggest that Polycomb group proteins Eed and Ezh2 functionally interact in mammalian cells, an interaction that is mediated by the WD40-containing domain of Eed protein.
Figures



Similar articles
-
The product of the murine homolog of the Drosophila extra sex combs gene displays transcriptional repressor activity.Mol Cell Biol. 1997 Aug;17(8):4707-17. doi: 10.1128/MCB.17.8.4707. Mol Cell Biol. 1997. PMID: 9234727 Free PMC article.
-
Characterization of interactions between the mammalian polycomb-group proteins Enx1/EZH2 and EED suggests the existence of different mammalian polycomb-group protein complexes.Mol Cell Biol. 1998 Jun;18(6):3586-95. doi: 10.1128/MCB.18.6.3586. Mol Cell Biol. 1998. PMID: 9584199 Free PMC article.
-
Structural basis of EZH2 recognition by EED.Structure. 2007 Oct;15(10):1306-15. doi: 10.1016/j.str.2007.08.007. Structure. 2007. PMID: 17937919
-
Polycomb repressive complex's evolutionary conserved function: the role of EZH2 status and cellular background.Clin Epigenetics. 2016 May 27;8:55. doi: 10.1186/s13148-016-0226-1. eCollection 2016. Clin Epigenetics. 2016. PMID: 27239242 Free PMC article. Review.
-
PRC2-complex related dysfunction in overgrowth syndromes: A review of EZH2, EED, and SUZ12 and their syndromic phenotypes.Am J Med Genet C Semin Med Genet. 2019 Dec;181(4):519-531. doi: 10.1002/ajmg.c.31754. Epub 2019 Nov 14. Am J Med Genet C Semin Med Genet. 2019. PMID: 31724824 Review.
Cited by
-
Genetics of MDS.Blood. 2019 Mar 7;133(10):1049-1059. doi: 10.1182/blood-2018-10-844621. Epub 2019 Jan 22. Blood. 2019. PMID: 30670442 Free PMC article. Review.
-
Functions of noncoding RNAs in neural development and neurological diseases.Mol Neurobiol. 2011 Dec;44(3):359-73. doi: 10.1007/s12035-011-8211-3. Epub 2011 Oct 4. Mol Neurobiol. 2011. PMID: 21969146 Free PMC article. Review.
-
Expression and clinicopathological significance of EED, SUZ12 and EZH2 mRNA in colorectal cancer.J Cancer Res Clin Oncol. 2015 Apr;141(4):661-9. doi: 10.1007/s00432-014-1854-5. Epub 2014 Oct 19. J Cancer Res Clin Oncol. 2015. PMID: 25326896
-
PRC2 is required for extensive reorganization of H3K27me3 during epigenetic reprogramming in mouse fetal germ cells.Epigenetics Chromatin. 2017 Feb 20;10:7. doi: 10.1186/s13072-017-0113-9. eCollection 2017. Epigenetics Chromatin. 2017. PMID: 28239420 Free PMC article.
-
A Drosophila ESC-E(Z) protein complex is distinct from other polycomb group complexes and contains covalently modified ESC.Mol Cell Biol. 2000 May;20(9):3069-78. doi: 10.1128/MCB.20.9.3069-3078.2000. Mol Cell Biol. 2000. PMID: 10757791 Free PMC article.
References
-
- Abel K J, Brody L C, Vlades J M, Erdos M R, McKinley D R, Castilla L H, Merajver S D, Couch F J, Friedman L S, Ostermeyer E A, Lynch E D, King M-C, Welcsh P L, Osborne-Lawrence S, Spillman M, Bowcock A M, Collins F S, Weber B L. Characterization of EZH1, a human homolog of Drosophila Enhancer of zeste near BRCA1. Genomics. 1996;37:161–171. - PubMed
-
- Bird T A, Schule H, Delaney P B, Sims J E, Thoma B, Dower S K. Evidence that MAP (mitogen-activated protein) kinase activation may be a necessary but not sufficient signal for a restricted subset of responses in IL-1-treated epidermoid cells. Cytokine. 1992;4:429–440. - PubMed
-
- Bomsztyk K, Van Seuningen I, Suzuki H, Denisenko O, Ostrowski J. Diverse molecular interactions of the hnRNP K protein. FEBS Lett. 1997;403:113–115. - PubMed
-
- Burd C G, Dreyfuss G. Conserved structures and diversity of functions of RNA-binding proteins. Science. 1994;265:615–621. - PubMed
-
- Carrington E A, Jones R S. The Drosophila Enhancer of zeste gene encodes a chromatin protein: examination of wild-type and mutant protein distribution. Development. 1996;122:4073–4083. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
