Tobacco mosaic virus infection induces severe morphological changes of the endoplasmic reticulum
- PMID: 9736708
- PMCID: PMC21614
- DOI: 10.1073/pnas.95.19.11169
Tobacco mosaic virus infection induces severe morphological changes of the endoplasmic reticulum
Abstract
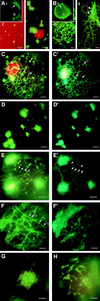
The tobacco mosaic virus (TMV) movement protein (MP) facilitates transport of virus infection between adjacent cells by modifying plasmodesmata. Previous studies suggested that the cytoskeleton and the endomembrane system are involved in this transport. We examined the effects of TMV infection on the endoplasmic reticulum (ER) in transgenic Nicotiana benthamiana that accumulate the green fluorescent protein (GFP) in the ER. Fluorescence microscopy was used to show that early in infection the ER undergoes dramatic morphological changes that include the conversion of tubular ER into large aggregates that revert to tubular ER in later stages of infection. These changes parallel MP accumulation and degradation. Furthermore, a fusion protein comprising MP fused to GFP accumulates in or on these large aggregates of ER. Expression of MP-GFP in the absence of virus infection led to the production of fluorescent aggregates of the same apparent form and size. Microsomes isolated from infected leaves contain MP. We show that the MP appears to behave as an integral ER membrane protein and is exposed on the cytosolic face of the ER. The importance of the association of MP with ER and its possible role in intracellular and intercellular spread of infection is discussed.
Figures




Similar articles
-
Changing patterns of localization of the tobacco mosaic virus movement protein and replicase to the endoplasmic reticulum and microtubules during infection.Plant Cell. 1998 Jul;10(7):1107-20. doi: 10.1105/tpc.10.7.1107. Plant Cell. 1998. PMID: 9668131 Free PMC article.
-
Tobacco mosaic virus (TMV) replicase and movement protein function synergistically in facilitating TMV spread by lateral diffusion in the plasmodesmal desmotubule of Nicotiana benthamiana.Mol Plant Microbe Interact. 2008 Mar;21(3):335-45. doi: 10.1094/MPMI-21-3-0335. Mol Plant Microbe Interact. 2008. PMID: 18257683
-
Cellular targets of functional and dysfunctional mutants of tobacco mosaic virus movement protein fused to green fluorescent protein.J Virol. 2000 Dec;74(23):11339-46. doi: 10.1128/jvi.74.23.11339-11346.2000. J Virol. 2000. PMID: 11070034 Free PMC article.
-
Role of P30 in replication and spread of TMV.Traffic. 2000 Jul;1(7):540-4. doi: 10.1034/j.1600-0854.2000.010703.x. Traffic. 2000. PMID: 11208141 Review.
-
Tobacco mosaic virus: a pioneer of cell-to-cell movement.Philos Trans R Soc Lond B Biol Sci. 1999 Mar 29;354(1383):637-43. doi: 10.1098/rstb.1999.0415. Philos Trans R Soc Lond B Biol Sci. 1999. PMID: 10212943 Free PMC article. Review.
Cited by
-
Interactions of the TGB1 protein during cell-to-cell movement of Barley stripe mosaic virus.J Virol. 2001 Sep;75(18):8712-23. doi: 10.1128/jvi.75.18.8712-8723.2001. J Virol. 2001. PMID: 11507216 Free PMC article.
-
Three-Dimensional Architecture and Biogenesis of Membrane Structures Associated with Plant Virus Replication.Front Plant Sci. 2018 Jan 30;9:57. doi: 10.3389/fpls.2018.00057. eCollection 2018. Front Plant Sci. 2018. PMID: 29441085 Free PMC article. Review.
-
Replication of tobacco mosaic virus on endoplasmic reticulum and role of the cytoskeleton and virus movement protein in intracellular distribution of viral RNA.J Cell Biol. 1999 Nov 29;147(5):945-58. doi: 10.1083/jcb.147.5.945. J Cell Biol. 1999. PMID: 10579716 Free PMC article.
-
Cell-to-cell trafficking of RNA and RNA silencing through plasmodesmata.Protoplasma. 2011 Jan;248(1):101-16. doi: 10.1007/s00709-010-0225-6. Epub 2010 Nov 2. Protoplasma. 2011. PMID: 21042816 Review.
-
The Tobacco mosaic virus movement protein associates with but does not integrate into biological membranes.J Virol. 2014 Mar;88(5):3016-26. doi: 10.1128/JVI.03648-13. Epub 2013 Dec 26. J Virol. 2014. PMID: 24371064 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

