A protein kinase, PKN, accumulates in Alzheimer neurofibrillary tangles and associated endoplasmic reticulum-derived vesicles and phosphorylates tau protein
- PMID: 9736660
- PMCID: PMC6793236
- DOI: 10.1523/JNEUROSCI.18-18-07402.1998
A protein kinase, PKN, accumulates in Alzheimer neurofibrillary tangles and associated endoplasmic reticulum-derived vesicles and phosphorylates tau protein
Abstract
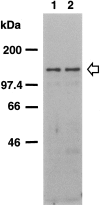
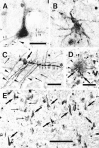
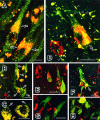
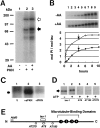
A possible role for a protein kinase, PKN, a fatty acid-activated serine/threonine kinase with a catalytic domain homologous to the protein kinase C family and a direct target for Rho, was investigated in the pathology of Alzheimer's disease (AD) using a sensitive immunocytochemistry on postmortem human brain tissues and a kinase assay for human tau protein. The present study provides evidences by light, electron, and confocal laser microscopy that in control human brains, PKN is enriched in neurons, where the kinase is concentrated in a subset of endoplasmic reticulum (ER) and ER-derived vesicles localized to the apical compartment of juxtanuclear cytoplasm, as well as late endosomes, multivesicular bodies, Golgi bodies, secretary vesicles, and nuclei. In AD-affected neurons, PKN was redistributed to the cortical cytoplasm and neurites and was closely associated with neurofibrillary tangles (NFTs) and their major constituent, abnormally modified tau. PKN was also found in degenerative neurites within senile plaques. In addition, we report that human tau protein is directly phosphorylated by PKN both in vitro and in vivo. Thus, our results suggest a specific role for PKN in NFT formation and neurodegeneration in AD damaged neurons.
Figures

Similar articles
-
A peptidyl-prolyl isomerase, FKBP12, accumulates in Alzheimer neurofibrillary tangles.Neurosci Lett. 2009 Aug 7;459(2):96-9. doi: 10.1016/j.neulet.2009.04.062. Epub 2009 May 3. Neurosci Lett. 2009. PMID: 19414059
-
mTor mediates tau localization and secretion: Implication for Alzheimer's disease.Biochim Biophys Acta. 2015 Jul;1853(7):1646-57. doi: 10.1016/j.bbamcr.2015.03.003. Epub 2015 Mar 17. Biochim Biophys Acta. 2015. PMID: 25791428
-
S6 kinase phosphorylated at T229 is involved in tau and actin pathologies in Alzheimer's disease.Neuropathology. 2016 Aug;36(4):325-32. doi: 10.1111/neup.12275. Epub 2015 Nov 18. Neuropathology. 2016. PMID: 26582459
-
Current advances on different kinases involved in tau phosphorylation, and implications in Alzheimer's disease and tauopathies.Curr Alzheimer Res. 2005 Jan;2(1):3-18. doi: 10.2174/1567205052772713. Curr Alzheimer Res. 2005. PMID: 15977985 Review.
-
Mechanisms of neurofibrillary degeneration and the formation of neurofibrillary tangles.J Neural Transm Suppl. 1998;53:169-80. doi: 10.1007/978-3-7091-6467-9_15. J Neural Transm Suppl. 1998. PMID: 9700655 Review.
Cited by
-
Fragmentation of protein kinase N (PKN) in the hydrocephalic rat brain.Acta Histochem Cytochem. 2007 Aug 30;40(4):113-21. doi: 10.1267/ahc.07011. Acta Histochem Cytochem. 2007. PMID: 17898875 Free PMC article.
-
Development of an intracellularly acting inhibitory peptide selective for PKN.Biochem J. 2009 Dec 23;425(2):445-53. doi: 10.1042/BJ20090380. Biochem J. 2009. PMID: 19857203 Free PMC article.
-
Rapid induction of intraneuronal neurofibrillary tangles in apolipoprotein E-deficient mice.Proc Natl Acad Sci U S A. 2001 Jul 17;98(15):8832-7. doi: 10.1073/pnas.151253098. Epub 2001 Jul 3. Proc Natl Acad Sci U S A. 2001. PMID: 11438710 Free PMC article.
-
Modulation of tau phosphorylation and intracellular localization by cellular stress.Biochem J. 2000 Jan 15;345 Pt 2(Pt 2):263-70. Biochem J. 2000. PMID: 10620503 Free PMC article.
-
Protein kinase N1 critically regulates cerebellar development and long-term function.J Clin Invest. 2018 May 1;128(5):2076-2088. doi: 10.1172/JCI96165. Epub 2018 Apr 16. J Clin Invest. 2018. PMID: 29494346 Free PMC article.
References
-
- Alafuzoff I, Iqbal K, Friden H, Adolfsson R, Winblad B. Histopathological criteria for progressive dementia disorders: clinico-pathological correlation and classification by multivariate data analysis. Acta Neuropathol (Berl) 1987;74:209–225. - PubMed
-
- Amano M, Mukai H, Ono Y, Chihara K, Matsui T, Hamajima Y, Okawa K, Iwamatsu A, Kaibuchi K. Identification of a putative target for Rho as the serine-threonine kinase protein kinase N. Science. 1996;271:648–650. - PubMed
-
- Bancher C, Grundke-Iqbal I, Iqbal K, Fried VA, Smith HT, Wisniewski HM. Abnormal phosphorylation of tau preceded ubiquitination in neurofibrillary pathology of Alzheimer disease. Brain Res. 1991;539:11–18. - PubMed
-
- Carmel G, Mager EM, Binder LI, Kuret J. The structural basis of monoclonal antibody Alz50’s selectivity for Alzheimer’s disease pathology. J Biol Chem. 1996;271:32789–32795. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
