Activity of the delta-opioid receptor is partially reduced, whereas activity of the kappa-receptor is maintained in mice lacking the mu-receptor
- PMID: 9736649
- PMCID: PMC6793240
- DOI: 10.1523/JNEUROSCI.18-18-07285.1998
Activity of the delta-opioid receptor is partially reduced, whereas activity of the kappa-receptor is maintained in mice lacking the mu-receptor
Abstract
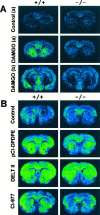
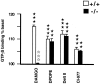
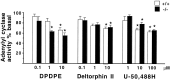
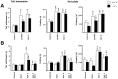
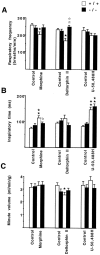
Previous pharmacological studies have indicated the possible existence of functional interactions between mu-, delta- and kappa-opioid receptors in the CNS. We have investigated this issue using a genetic approach. Here we describe in vitro and in vivo functional activity of delta- and kappa-opioid receptors in mice lacking the mu-opioid receptor (MOR). Measurements of agonist-induced [35S]GTPgammaS binding and adenylyl cyclase inhibition showed that functional coupling of delta- and kappa-receptors to G-proteins is preserved in the brain of mutant mice. In the mouse vas deferens bioassay, deltorphin II and cyclic[D-penicillamine2, D-penicillamine5] enkephalin exhibited similar potency to inhibit smooth muscle contraction in both wild-type and MOR -/- mice. delta-Analgesia induced by deltorphin II was slightly diminished in mutant mice, when the tail flick test was used. Deltorphin II strongly reduced the respiratory frequency in wild-type mice but not in MOR -/- mice. Analgesic and respiratory responses produced by the selective kappa-agonist U-50,488H were unchanged in MOR-deficient mice. In conclusion, the preservation of delta- and kappa-receptor signaling properties in mice lacking mu-receptors provides no evidence for opioid receptor cross-talk at the cellular level. Intact antinociceptive and respiratory responses to the kappa-agonist further suggest that the kappa-receptor mainly acts independently from the mu-receptor in vivo. Reduced delta-analgesia and the absence of delta-respiratory depression in MOR-deficient mice together indicate that functional interactions may take place between mu-receptors and central delta-receptors in specific neuronal pathways.
Figures

Similar articles
-
Stimulation of phospholipase C by the cloned mu, delta and kappa opioid receptors via chimeric G alpha(q) mutants.Eur J Neurosci. 1999 Feb;11(2):383-8. doi: 10.1046/j.1460-9568.1999.00442.x. Eur J Neurosci. 1999. PMID: 10051738
-
Absence of G-protein activation by mu-opioid receptor agonists in the spinal cord of mu-opioid receptor knockout mice.Br J Pharmacol. 1999 Jan;126(2):451-6. doi: 10.1038/sj.bjp.0702330. Br J Pharmacol. 1999. PMID: 10077238 Free PMC article.
-
Activity of mu- and delta-opioid agonists in vas deferens from mice deficient in MOR gene.Br J Pharmacol. 2001 Apr;132(7):1485-92. doi: 10.1038/sj.bjp.0703966. Br J Pharmacol. 2001. PMID: 11264242 Free PMC article.
-
Biased Opioid Ligands.Molecules. 2020 Sep 16;25(18):4257. doi: 10.3390/molecules25184257. Molecules. 2020. PMID: 32948048 Free PMC article. Review.
-
Relevance of Mu-Opioid Receptor Splice Variants and Plasticity of Their Signaling Sequelae to Opioid Analgesic Tolerance.Cell Mol Neurobiol. 2021 Jul;41(5):855-862. doi: 10.1007/s10571-020-00934-y. Epub 2020 Aug 17. Cell Mol Neurobiol. 2021. PMID: 32804312 Review.
Cited by
-
Peptide-derived ligands for the discovery of safer opioid analgesics.Drug Discov Today. 2024 May;29(5):103950. doi: 10.1016/j.drudis.2024.103950. Epub 2024 Mar 20. Drug Discov Today. 2024. PMID: 38514040 Free PMC article. Review.
-
Mu and Delta opioid receptors activate the same G proteins in human neuroblastoma SH-SY5Y cells.Br J Pharmacol. 2002 Jan;135(1):217-25. doi: 10.1038/sj.bjp.0704430. Br J Pharmacol. 2002. PMID: 11786497 Free PMC article.
-
Opioid-Induced Pronociceptive Signaling in the Gastrointestinal Tract Is Mediated by Delta-Opioid Receptor Signaling.J Neurosci. 2022 Apr 20;42(16):3316-3328. doi: 10.1523/JNEUROSCI.2098-21.2022. Epub 2022 Mar 7. J Neurosci. 2022. PMID: 35256532 Free PMC article.
-
Recent advances on the δ opioid receptor: from trafficking to function.Br J Pharmacol. 2015 Jan;172(2):403-19. doi: 10.1111/bph.12706. Epub 2014 Jul 1. Br J Pharmacol. 2015. PMID: 24665909 Free PMC article. Review.
-
Controlling opioid receptor functional selectivity by targeting distinct subpockets of the orthosteric site.Elife. 2021 Feb 8;10:e56519. doi: 10.7554/eLife.56519. Elife. 2021. PMID: 33555255 Free PMC article.
References
-
- Baamonde A, Dauge V, Ruiz-Gayo M, Fulga IG, Turcaud S, Fournie-Zaluski M-C, Roques BP. Antidepressant-type effects of endogenous enkephalins protected by systemic RB 101 are mediated by opioid δ and dopamine D1 receptor stimulation. Eur J Pharmacol. 1992;216:157–166. - PubMed
-
- Bartlett D, Jr, Tenney SM. Control of breathing in experimental anemia. Respir Physiol. 1970;10:384–395. - PubMed
-
- Corbett AD, Paterson SJ, Kosterlitz HW. Selectivity of ligands for opioid receptors. In: Herz A, editor. Handbook of experimental pharmacology, Vol 104, Opioids I. Springer; New York: 1993. pp. 645–679.
-
- Cowan A, Zhu XZ, Mosberg HI, Omnaas JR, Porreca F. Direct dependence studies in rats with agents selective for different types of opioid receptors. J Pharmacol Exp Ther. 1988;246:950–955. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
