Functional expression of the Wilson disease protein reveals mislocalization and impaired copper-dependent trafficking of the common H1069Q mutation
- PMID: 9724794
- PMCID: PMC27985
- DOI: 10.1073/pnas.95.18.10854
Functional expression of the Wilson disease protein reveals mislocalization and impaired copper-dependent trafficking of the common H1069Q mutation
Abstract
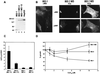
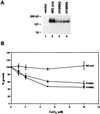
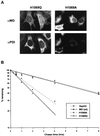
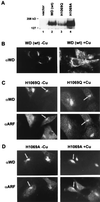
Wilson disease is an autosomal recessive disorder of hepatic copper metabolism caused by mutations in a gene encoding a copper-transporting P-type ATPase. To elucidate the function of the Wilson protein, wild-type and mutant Wilson cDNAs were expressed in a Menkes copper transporter-deficient mottled fibroblast cell line defective in copper export. Expression of the wild-type cDNA demonstrated trans-Golgi network localization and copper-dependent trafficking of the Wilson protein identical to previous observations for the endogenously expressed protein in hepatocytes. Furthermore, expression of the Wilson cDNA rescued the mottled phenotype as evidenced by a reduction in copper accumulation and restoration of cell viability. In contrast, expression of an H1069Q mutant Wilson cDNA did not rescue the mottled phenotype, and immunofluorescence studies showed that this mutant Wilson protein was localized in the endoplasmic reticulum. Consistent with these findings, pulse-chase analysis demonstrated a 5-fold decrease in the half-life of the H1069Q mutant as compared with the wild-type protein. Maintenance of these transfected cell lines at 28 degreesC resulted in localization of the H1069Q protein in the trans-Golgi network, suggesting that a temperature-sensitive defect in protein folding followed by degradation constitutes the molecular basis of Wilson disease in patients harboring the H1069Q mutation. Taken together, these studies describe a tractable expression system for elucidating the function and localization of the copper-transporting ATPases in mammalian cells and provide compelling evidence that the Wilson protein can functionally substitute for the Menkes protein, supporting the concept that these proteins use common biochemical mechanisms to effect cellular copper homeostasis.
Figures
Similar articles
-
Effect of the toxic milk mutation (tx) on the function and intracellular localization of Wnd, the murine homologue of the Wilson copper ATPase.Hum Mol Genet. 2001 Feb 15;10(4):361-70. doi: 10.1093/hmg/10.4.361. Hum Mol Genet. 2001. PMID: 11157799
-
Functional characterization of missense mutations in ATP7B: Wilson disease mutation or normal variant?Am J Hum Genet. 1998 Dec;63(6):1663-74. doi: 10.1086/302163. Am J Hum Genet. 1998. PMID: 9837819 Free PMC article.
-
Analysis of functional domains of Wilson disease protein (ATP7B) in Saccharomyces cerevisiae.FEBS Lett. 1998 May 29;428(3):281-5. doi: 10.1016/s0014-5793(98)00546-8. FEBS Lett. 1998. PMID: 9654149
-
Pathophysiology and clinical features of Wilson disease.Metab Brain Dis. 2004 Dec;19(3-4):229-39. doi: 10.1023/b:mebr.0000043973.10494.85. Metab Brain Dis. 2004. PMID: 15554419 Review.
-
[Wilson disease].Orv Hetil. 2004 Oct 17;145(42):2147-51. Orv Hetil. 2004. PMID: 15566072 Review. Hungarian.
Cited by
-
p.P1379S, a benign variant with reduced ATP7B protein level in Wilson Disease.JIMD Rep. 2020 May 19;54(1):32-36. doi: 10.1002/jmd2.12127. eCollection 2020 Jul. JIMD Rep. 2020. PMID: 32685348 Free PMC article.
-
Copper transporting P-type ATPases and human disease.J Bioenerg Biomembr. 2002 Oct;34(5):333-8. doi: 10.1023/a:1021293818125. J Bioenerg Biomembr. 2002. PMID: 12539960 Review.
-
Identifying Differentially Expressed MicroRNAs, Target Genes, and Key Pathways Deregulated in Patients with Liver Diseases.Int J Mol Sci. 2020 Oct 6;21(19):7368. doi: 10.3390/ijms21197368. Int J Mol Sci. 2020. PMID: 33036164 Free PMC article.
-
Hepatocellular transport proteins and their role in liver disease.World J Gastroenterol. 2001 Apr;7(2):157-69. doi: 10.3748/wjg.v7.i2.157. World J Gastroenterol. 2001. PMID: 11819755 Free PMC article. Review. No abstract available.
-
An αB-Crystallin Peptide Rescues Compartmentalization and Trafficking Response to Cu Overload of ATP7B-H1069Q, the Most Frequent Cause of Wilson Disease in the Caucasian Population.Int J Mol Sci. 2018 Jun 27;19(7):1892. doi: 10.3390/ijms19071892. Int J Mol Sci. 2018. PMID: 29954118 Free PMC article.
References
-
- Cox D W. Prog Liver Dis. 1996;14:245–264. - PubMed
-
- Cuthbert, J. A. (1998) Gastroenterol. Clin. N. Amer. 27, in press. - PubMed
-
- Bull P C, Thomas G R, Rommens J M, Forbes J R, Cox D W. Nat Genet. 1993;5:327–337. - PubMed
-
- Tanzi R E, Petrukhin K, Chernov I, Pellequer J L, Wasco W, Ross B, Romano D M, Parano E, Pavone L, Brzustowicz L M, et al. Nat Genet. 1993;5:344–350. - PubMed
-
- Yamaguchi Y, Heiny M E, Gitlin J D. Biochem Biophys Res Commun. 1993;197:271–277. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

