Generation of mucosal cytotoxic T cells against soluble protein by tissue-specific environmental and costimulatory signals
- PMID: 9724787
- PMCID: PMC27978
- DOI: 10.1073/pnas.95.18.10814
Generation of mucosal cytotoxic T cells against soluble protein by tissue-specific environmental and costimulatory signals
Abstract
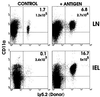
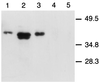
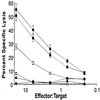
We compared peripheral and mucosal primary CD8 T cell responses to inflammatory and noninflammatory forms of antigen in a T cell-adoptive transfer system. Immunization with the soluble antigen, ovalbumin (ova), administered i.p. or orally without adjuvant, activated nonmucosal CD8 T cells but did not induce cytotoxic activity. However, after activation, the transferred cells entered the intestinal mucosa and became potent antigen-specific killers. Thus, exogenous intact soluble protein entered the major histocompatibility complex class I antigen presentation pathway and induced mucosal cytotoxic T lymphocytes. Moreover, distinct costimulatory requirements for activation of peripheral versus mucosal T cells were noted in that the CD28 ligand, B7-1, was critical for activated mucosal T cell generation but not for activation of peripheral CD8 T cells. The costimulator, B7-2, was required for optimum activation of both populations. Infection with a new recombinant vesicular stomatitis virus encoding ovalbumin induced lytic activity in mucosal as well as peripheral sites, demonstrating an adjuvant effect of inflammatory mediators produced during virus infection. Generation of antiviral cytotoxic T lymphocytes was also costimulation-dependent. The results indicated that induction of peripheral tolerance via antigen administration may not extend to mucosal sites because of distinct costimulatory and inflammatory signals in the mucosa.
Figures



Similar articles
-
Soluble antigen and CD40 triggering are sufficient to induce primary and memory cytotoxic T cells.J Immunol. 2000 Jan 15;164(2):725-32. doi: 10.4049/jimmunol.164.2.725. J Immunol. 2000. PMID: 10623816
-
The role of B7-1 and B7-2 costimulation for the generation of CTL responses in vivo.J Immunol. 1998 Sep 15;161(6):2740-5. J Immunol. 1998. PMID: 9743331
-
B7 costimulation is necessary for the activation of the lytic function in cytotoxic T lymphocyte precursors.J Immunol. 1995 Dec 1;155(11):5167-74. J Immunol. 1995. PMID: 7594526
-
Lentiviral Protein Transfer Vectors Are an Efficient Vaccine Platform and Induce a Strong Antigen-Specific Cytotoxic T Cell Response.J Virol. 2015 Sep;89(17):9044-60. doi: 10.1128/JVI.00844-15. Epub 2015 Jun 17. J Virol. 2015. PMID: 26085166 Free PMC article.
-
Induction and visualization of mucosal memory CD8 T cells following systemic virus infection.J Immunol. 1999 Oct 15;163(8):4125-32. J Immunol. 1999. PMID: 10510347
Cited by
-
Tissue-resident memory T cells break tolerance to renal autoantigens and orchestrate immune-mediated nephritis.Cell Mol Immunol. 2024 Sep;21(9):1066-1081. doi: 10.1038/s41423-024-01197-z. Epub 2024 Jul 3. Cell Mol Immunol. 2024. PMID: 38961265 Free PMC article.
-
Combined IL-15/IL-15Ralpha immunotherapy maximizes IL-15 activity in vivo.J Immunol. 2006 Nov 1;177(9):6072-80. doi: 10.4049/jimmunol.177.9.6072. J Immunol. 2006. PMID: 17056533 Free PMC article.
-
IL-15R alpha expression on CD8+ T cells is dispensable for T cell memory.Proc Natl Acad Sci U S A. 2003 Apr 15;100(8):4724-9. doi: 10.1073/pnas.0737048100. Epub 2003 Apr 1. Proc Natl Acad Sci U S A. 2003. PMID: 12671073 Free PMC article.
-
Exploring regulatory mechanisms of CD8+ T cell contraction.Proc Natl Acad Sci U S A. 2008 Oct 28;105(43):16689-94. doi: 10.1073/pnas.0808997105. Epub 2008 Oct 22. Proc Natl Acad Sci U S A. 2008. PMID: 18946035 Free PMC article.
-
NFκB-Pim-1-Eomesodermin axis is critical for maintaining CD8 T-cell memory quality.Proc Natl Acad Sci U S A. 2017 Feb 28;114(9):E1659-E1667. doi: 10.1073/pnas.1608448114. Epub 2017 Feb 13. Proc Natl Acad Sci U S A. 2017. PMID: 28193872 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

