Complement depletion reduces macrophage infiltration and activation during Wallerian degeneration and axonal regeneration
- PMID: 9712643
- PMCID: PMC6792968
- DOI: 10.1523/JNEUROSCI.18-17-06713.1998
Complement depletion reduces macrophage infiltration and activation during Wallerian degeneration and axonal regeneration
Abstract
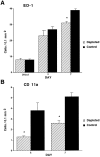
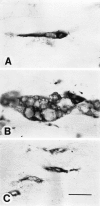
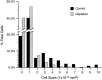
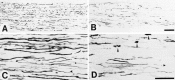
After peripheral nerve injury, macrophages infiltrate the degenerating nerve and participate in the removal of myelin and axonal debris, in Schwann cell proliferation, and in axonal regeneration. In vitro studies have demonstrated the role serum complement plays in both macrophage invasion and activation during Wallerian degeneration of peripheral nerve. To determine its role in vivo, we depleted serum complement for 1 week in adult Lewis rats, using intravenously administered cobra venom factor. At 1 d after complement depletion the right sciatic nerve was crushed, and the animals were sacrificed 4 and 7 d later. Macrophage identification with ED-1 and CD11a monoclonal antibodies revealed a significant reduction in their recruitment into distal degenerating nerve in complement-depleted animals. Complement depletion also decreased macrophage activation, as indicated by their failure to become large and multivacuolated and their reduced capacity to clear myelin, which was evident at both light and electron microscopic levels. Axonal regeneration was delayed in complement-depleted animals. These findings support a role for serum complement in both the recruitment and activation of macrophages during peripheral nerve degeneration as well as a role for macrophages in promoting axonal regeneration.
Figures



Similar articles
-
Differential macrophage responses in the peripheral and central nervous system during wallerian degeneration of axons.Exp Neurol. 1995 Dec;136(2):183-98. doi: 10.1006/exnr.1995.1095. Exp Neurol. 1995. PMID: 7498408
-
Deletion of SIRPα (signal regulatory protein-α) promotes phagocytic clearance of myelin debris in Wallerian degeneration, axon regeneration, and recovery from nerve injury.J Neuroinflammation. 2019 Dec 28;16(1):277. doi: 10.1186/s12974-019-1679-x. J Neuroinflammation. 2019. PMID: 31883525 Free PMC article.
-
Blocking of up-regulated ICAM-1 does not prevent macrophage infiltration during Wallerian degeneration of peripheral nerve.Exp Neurol. 2004 Jun;187(2):430-44. doi: 10.1016/j.expneurol.2004.02.004. Exp Neurol. 2004. PMID: 15144869
-
Role of macrophages in Wallerian degeneration and axonal regeneration after peripheral nerve injury.Acta Neuropathol. 2015 Nov;130(5):605-18. doi: 10.1007/s00401-015-1482-4. Epub 2015 Sep 29. Acta Neuropathol. 2015. PMID: 26419777 Review.
-
The role of macrophages in Wallerian degeneration.Brain Pathol. 1997 Apr;7(2):741-52. doi: 10.1111/j.1750-3639.1997.tb01060.x. Brain Pathol. 1997. PMID: 9161725 Free PMC article. Review.
Cited by
-
Activation of innate and humoral immunity in the peripheral nervous system of ALS transgenic mice.Proc Natl Acad Sci U S A. 2009 Dec 8;106(49):20960-5. doi: 10.1073/pnas.0911405106. Epub 2009 Nov 20. Proc Natl Acad Sci U S A. 2009. PMID: 19933335 Free PMC article.
-
Understanding painful versus non-painful dental pain in female and male patients: A transcriptomic analysis of human biopsies.PLoS One. 2023 Sep 21;18(9):e0291724. doi: 10.1371/journal.pone.0291724. eCollection 2023. PLoS One. 2023. PMID: 37733728 Free PMC article.
-
Deficiency in complement C1q improves histological and functional locomotor outcome after spinal cord injury.J Neurosci. 2008 Dec 17;28(51):13876-88. doi: 10.1523/JNEUROSCI.2823-08.2008. J Neurosci. 2008. PMID: 19091977 Free PMC article.
-
Deciphering peripheral nerve myelination by using Schwann cell expression profiling.Proc Natl Acad Sci U S A. 2002 Jun 25;99(13):8998-9003. doi: 10.1073/pnas.132080999. Proc Natl Acad Sci U S A. 2002. PMID: 12084938 Free PMC article.
-
The membrane attack complex of the complement system is essential for rapid Wallerian degeneration.J Neurosci. 2007 Jul 18;27(29):7663-72. doi: 10.1523/JNEUROSCI.5623-06.2007. J Neurosci. 2007. PMID: 17634361 Free PMC article.
References
-
- Avellino AM, Hart D, Dailey AT, MacKinnon M, Ellegala D, Kliot M. Differential macrophage responses in the peripheral and central nervous system during Wallerian degeneration of axons. Exp Neurol. 1995;136:183–198. - PubMed
-
- Bedi KS, Winter J, Berry M, Cohen J. Adult rat dorsal root ganglion neurons extend neurites on predegenerated but not on normal peripheral nerves in vitro. Eur J Neurosci. 1992;4:193–200. - PubMed
-
- Beuche W, Friede RL. The role of non-resident cells in Wallerian degeneration. J Neurocytol. 1984;13:767–796. - PubMed
-
- Beuche W, Friede RL. Myelin phagocytosis in Wallerian degeneration depends on silica-sensitive, bg/bg-negative and Fc-positive monocytes. Brain Res. 1986;378:97–106. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
