Production of vascular endothelial growth factor by murine macrophages: regulation by hypoxia, lactate, and the inducible nitric oxide synthase pathway
- PMID: 9708818
- PMCID: PMC1852978
- DOI: 10.1016/S0002-9440(10)65601-5
Production of vascular endothelial growth factor by murine macrophages: regulation by hypoxia, lactate, and the inducible nitric oxide synthase pathway
Abstract
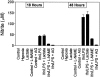
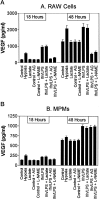
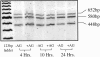
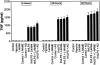
Murine thioglycolate-induced peritoneal macrophages (MPMs) and the murine RAW264.7 macrophage-like cell line (RAW cells) constitutively produce vascular endothelial growth factor (VEGF). VEGF production is increased under hypoxic conditions or after cell activation with interferon-gamma (IFNgamma) and endotoxin (lipopolysaccharide, LPS). In contrast, tumor necrosis factor-alpha is produced only by IFNgamma/LPS-activated cells. Lactate (25 mmol/L) does not increase VEGF production by these cells. However, hypoxia, lactate, and IFNgamma/LPS-activated MPMs express angiogenic activity, whereas normoxic, nonactivated MPMs do not. Lack of angiogenic activity is not due to an antiangiogenic factor(s) in the medium of these cells. Angiogenic activity produced by hypoxia and lactate-treated MPMs is neutralized by anti-VEGF antibody, which also neutralizes most of the angiogenic activity produced by IFNgamma/LPS-activated MPMs. The inducible nitric oxide synthase inhibitors Ng-nitro-L-arginine-methyl ester (1.5 mmol/L) and aminoguanidine (1 mmol/L) block production of angiogenic activity by MPMs and RAW cells. In RAW cells, Ng-nitro-L-arginine-methyl ester and AG block IFNgamma/LPS-activated, but not constitutive, VEGF production, whereas in MPMs, neither constitutive nor IFNgamma/LPS-activated VEGF synthesis is affected. Synthesis of tumor necrosis factor-alpha is also unaffected. In contrast to normoxic, nonactivated MPMs, inducible nitric oxide synthase-inhibited, IFNgamma/LPS-activated MPMs produce an antiangiogenic factor(s). We conclude that VEGF is a major contributor to macrophage-derived angiogenic activity, and that activation by hypoxia, lactate, or IFNgamma/LPS switches macrophage-derived VEGF from a nonangiogenic to an angiogenic state. This switch may involve a posttranslational modification of VEGF, possibly by the process of ADP-ribosylation. ADP-ribosylation by MPM cytosolic extracts or by cholera toxin switches rVEGF165 from an angiogenic to a nonangiogenic state. In IFNgamma/LPS-activated MPMs, the inducible nitric oxide synthase-dependent pathway also regulates the expression of an antiangiogenic factor(s) that antagonizes the bioactivity of VEGF and provides an additional regulatory pathway controlling the angiogenic phenotype of macrophages.
Figures

Similar articles
-
Synergistic up-regulation of vascular endothelial growth factor expression in murine macrophages by adenosine A(2A) receptor agonists and endotoxin.Am J Pathol. 2002 Jun;160(6):2231-44. doi: 10.1016/S0002-9440(10)61170-4. Am J Pathol. 2002. PMID: 12057925 Free PMC article.
-
Regulation of vascular endothelial growth factor gene expression in murine macrophages by nitric oxide and hypoxia.Exp Biol Med (Maywood). 2003 Jun;228(6):697-705. doi: 10.1177/153537020322800608. Exp Biol Med (Maywood). 2003. PMID: 12773701
-
Nitric oxide synthase inhibition by N(G)-nitro-L-arginine methyl ester retards vascular sprouting in angiogenesis.Microvasc Res. 2003 Jan;65(1):2-8. doi: 10.1016/s0026-2862(02)00011-0. Microvasc Res. 2003. PMID: 12535865
-
Detection of Nitric Oxide Production by the Macrophage Cell Line RAW264.7: Version 2.2020 Sep. In: National Cancer Institute’s Nanotechnology Characterization Laboratory Assay Cascade Protocols [Internet]. Bethesda (MD): National Cancer Institute (US); 2005 May 1–. NCL Method ITA-7. 2020 Sep. In: National Cancer Institute’s Nanotechnology Characterization Laboratory Assay Cascade Protocols [Internet]. Bethesda (MD): National Cancer Institute (US); 2005 May 1–. NCL Method ITA-7. PMID: 39013045 Free Books & Documents. Review.
-
Enzyme-Linked Immunosorbent Assay (ELISA) for Detection of Human IFNγ in Culture Supernatants: Version 3.2020 Jun. In: National Cancer Institute’s Nanotechnology Characterization Laboratory Assay Cascade Protocols [Internet]. Bethesda (MD): National Cancer Institute (US); 2005 May 1–. NCL Method ITA-25. 2020 Jun. In: National Cancer Institute’s Nanotechnology Characterization Laboratory Assay Cascade Protocols [Internet]. Bethesda (MD): National Cancer Institute (US); 2005 May 1–. NCL Method ITA-25. PMID: 39013060 Free Books & Documents. Review.
Cited by
-
An angiogenic role for adrenomedullin in choroidal neovascularization.PLoS One. 2013;8(3):e58096. doi: 10.1371/journal.pone.0058096. Epub 2013 Mar 8. PLoS One. 2013. PMID: 23520487 Free PMC article.
-
Bone marrow-derived progenitor cells augment venous remodeling in a mouse dorsal skinfold chamber model.PLoS One. 2012;7(2):e32815. doi: 10.1371/journal.pone.0032815. Epub 2012 Feb 28. PLoS One. 2012. PMID: 22389724 Free PMC article.
-
Curcumin prophylaxis mitigates the incidence of hypobaric hypoxia-induced altered ion channels expression and impaired tight junction proteins integrity in rat brain.J Neuroinflammation. 2015 Jun 6;12:113. doi: 10.1186/s12974-015-0326-4. J Neuroinflammation. 2015. PMID: 26048285 Free PMC article.
-
The Stimulation of Macrophages by Systematical Administration of GM-CSF Can Accelerate Adult Wound Healing Process.Int J Mol Sci. 2022 Sep 25;23(19):11287. doi: 10.3390/ijms231911287. Int J Mol Sci. 2022. PMID: 36232590 Free PMC article.
-
Metabotropic glutamate receptor 5 mediates phosphorylation of vascular endothelial cadherin and nuclear localization of β-catenin in response to homocysteine.Vascul Pharmacol. 2012 Mar-Apr;56(3-4):159-67. doi: 10.1016/j.vph.2012.01.004. Epub 2012 Jan 21. Vascul Pharmacol. 2012. PMID: 22285407 Free PMC article.
References
-
- Polverini PJ, Cotran RS, Gimbrone MA, Jr, Unanue ER: Activated macrophages induce vascular proliferation. Nature 1977, 269:804-806 - PubMed
-
- Koch AE, Polverini PJ, Leibovich SJ: Induction of neovascularization by activated human monocytes. J Leukocyte Biol 1985, 37:279-288 - PubMed
-
- Polverini PJ: Macrophage-induced angiogenesis: a review. Cytokines 1989, 1:54-73
-
- Polverini PJ, Leibovich SJ: Induction of neovascularization and nonlymphoid mesenchymal cell proliferation by macrophage cell lines. Lab Invest 1985, 51:635-642 - PubMed
-
- Sunderkotter C, Steinbrink K, Goebeler M, Bhardwaj R, Sorg C: Macrophages and angiogenesis. J Leukocyte Biol 1994, 55:410-422 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

