Expression of autoactivated stromelysin-1 in mammary glands of transgenic mice leads to a reactive stroma during early development
- PMID: 9708806
- PMCID: PMC1852990
- DOI: 10.1016/S0002-9440(10)65589-7
Expression of autoactivated stromelysin-1 in mammary glands of transgenic mice leads to a reactive stroma during early development
Abstract
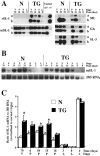
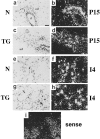
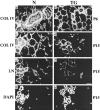
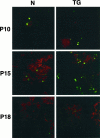
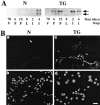
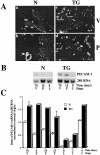
Extracellular matrix and extracellular matrix-degrading matrix metalloproteinases play a key role in interactions between the epithelium and the mesenchyme during mammary gland development and disease. In patients with breast cancer, the mammary mesenchyme undergoes a stromal reaction, the etiology of which is unknown. We previously showed that targeting of an autoactivating mutant of the matrix metalloproteinase stromelysin-1 to mammary epithelia of transgenic mice resulted in reduced mammary function during pregnancy and development of preneoplastic and neoplastic lesions. Here we examine the cascade of alterations before breast tumor formation in the mammary gland stroma once the expression of the stromelysin-1 transgene commences. Beginning in postpubertal virgin animals, low levels of transgene expression in mammary epithelia led to increased expression of endogenous stromelysin-1 in stromal fibroblasts and up-regulation of other matrix metalloproteinases, without basement membrane disruption. These changes were accompanied by the progressive development of a compensatory reactive stroma, characterized by increased collagen content and vascularization in glands from virgin mice. This remodeling of the gland affected epithelial-mesenchymal communication as indicated by inappropriate expression of tenascin-C starting by day 6 of pregnancy. This, together with increased transgene expression, led to basement membrane disruption starting by day 15 of pregnancy. We propose that the highly reactive stroma provides a prelude to breast epithelial tumors observed in these animals.
Figures

Similar articles
-
Matrix metalloproteinases are expressed during ductal and alveolar mammary morphogenesis, and misregulation of stromelysin-1 in transgenic mice induces unscheduled alveolar development.Mol Biol Cell. 1995 Oct;6(10):1287-303. doi: 10.1091/mbc.6.10.1287. Mol Biol Cell. 1995. PMID: 8573787 Free PMC article.
-
Targeted expression of stromelysin-1 in mammary gland provides evidence for a role of proteinases in branching morphogenesis and the requirement for an intact basement membrane for tissue-specific gene expression.J Cell Biol. 1994 May;125(3):681-93. doi: 10.1083/jcb.125.3.681. J Cell Biol. 1994. PMID: 8175886 Free PMC article.
-
Expression of stromelysin-1 and TIMP-1 in the involuting mammary gland and in early invasive tumors of the mouse.Int J Cancer. 1994 Nov 15;59(4):560-8. doi: 10.1002/ijc.2910590421. Int J Cancer. 1994. PMID: 7960227
-
The role of stromelysin-1 in stromal-epithelial interactions and cancer.Enzyme Protein. 1996;49(1-3):174-81. doi: 10.1159/000468624. Enzyme Protein. 1996. PMID: 8797005 Review.
-
Extracellular matrix remodeling and the regulation of epithelial-stromal interactions during differentiation and involution.Kidney Int Suppl. 1996 May;54:S68-74. Kidney Int Suppl. 1996. PMID: 8731199 Free PMC article. Review.
Cited by
-
The matrix metalloproteinase stromelysin-1 acts as a natural mammary tumor promoter.Oncogene. 2000 Feb 21;19(8):1102-13. doi: 10.1038/sj.onc.1203347. Oncogene. 2000. PMID: 10713697 Free PMC article. Review.
-
Stromelysin-1 regulates adipogenesis during mammary gland involution.J Cell Biol. 2001 Feb 19;152(4):693-703. doi: 10.1083/jcb.152.4.693. J Cell Biol. 2001. PMID: 11266461 Free PMC article.
-
Breast cancer by proxy: can the microenvironment be both the cause and consequence?Trends Mol Med. 2009 Jan;15(1):5-13. doi: 10.1016/j.molmed.2008.11.001. Epub 2008 Dec 16. Trends Mol Med. 2009. PMID: 19091631 Free PMC article.
-
Change in cell shape is required for matrix metalloproteinase-induced epithelial-mesenchymal transition of mammary epithelial cells.J Cell Biochem. 2008 Sep 1;105(1):25-33. doi: 10.1002/jcb.21821. J Cell Biochem. 2008. PMID: 18506791 Free PMC article.
-
Matrix metalloproteinase-induced genomic instability.Curr Opin Genet Dev. 2006 Feb;16(1):45-50. doi: 10.1016/j.gde.2005.12.011. Epub 2005 Dec 27. Curr Opin Genet Dev. 2006. PMID: 16377172 Free PMC article. Review.
References
-
- Van den Hooff A: Stromal involvement in malignant growth. Adv Cancer Res 1988, 50:159-196 - PubMed
-
- Sakakura T: New aspects of stroma-parenchyma relations in mammary gland differentiation. Int Rev Cytol 1991, 125:165-202 - PubMed
-
- Bissell MJ, Hall HG: Form and function in the mammary gland: the role of extracellular matrix. Neville M Daniel C eds. The Mammary Gland Development, Regulation and Function. 1987, :pp 97-146 Plenum Publishing Corp, New York
-
- Adams JC, Watt FM: Regulation of development and differentiation by the extracellular matrix. Development 1993, 117:1183-1198 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

