Functional analysis of the mouse Scn8a sodium channel
- PMID: 9698304
- PMCID: PMC6793187
- DOI: 10.1523/JNEUROSCI.18-16-06093.1998
Functional analysis of the mouse Scn8a sodium channel
Abstract
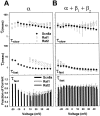
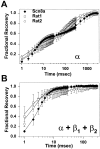
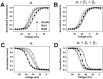
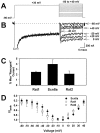
The mouse Scn8a sodium channel and its ortholog Na6 in the rat are abundantly expressed in the CNS. Mutations in mouse Scn8a result in neurological disorders, including paralysis, ataxia, and dystonia. In addition, Scn8a has been observed to mediate unique persistent and resurgent currents in cerebellar Purkinje cells (Raman et al., 1997). To examine the functional characteristics of this channel, we constructed a full-length cDNA clone encoding the mouse Scn8a sodium channel and expressed it in Xenopus oocytes. The electrophysiological properties of the Scn8a channels were compared with those of the Rat1 and Rat2 sodium channels. Scn8a channels were sensitive to tetrodotoxin at a level comparable to that of Rat1 or Rat2. Scn8a channels inactivated more rapidly and showed differences in their voltage-dependent properties compared with Rat1 and Rat2 when only the alpha subunits were expressed. Coexpression of the beta1 and beta2 subunits modulated the properties of Scn8a channels, but to a lesser extent than for the Rat1 or Rat2 channels. Therefore, all three channels showed similar voltage dependence and inactivation kinetics in the presence of the beta subunits. Scn8a channels coexpressed with the beta subunits exhibited a persistent current that became larger with increasing depolarization, which was not observed for either Rat1 or Rat2 channels. The unique persistent current observed for Scn8a channels is consistent with the hypothesis that this channel is responsible for distinct sodium conductances underlying repetitive firing of action potentials in Purkinje neurons.
Figures


Similar articles
-
Functional analysis of the rat I sodium channel in xenopus oocytes.J Neurosci. 1998 Feb 1;18(3):811-20. doi: 10.1523/JNEUROSCI.18-03-00811.1998. J Neurosci. 1998. PMID: 9437003 Free PMC article.
-
The absence of resurgent sodium current in mouse spinal neurons.Brain Res. 1999 Dec 4;849(1-2):162-8. doi: 10.1016/s0006-8993(99)02060-0. Brain Res. 1999. PMID: 10592298
-
The contribution of resurgent sodium current to high-frequency firing in Purkinje neurons: an experimental and modeling study.J Neurosci. 2003 Jun 15;23(12):4899-912. doi: 10.1523/JNEUROSCI.23-12-04899.2003. J Neurosci. 2003. PMID: 12832512 Free PMC article.
-
Allelic mutations of the sodium channel SCN8A reveal multiple cellular and physiological functions.Genetica. 2004 Sep;122(1):37-45. doi: 10.1007/s10709-004-1441-9. Genetica. 2004. PMID: 15619959 Review.
-
Sodium channels and neurological disease: insights from Scn8a mutations in the mouse.Neuroscientist. 2001 Apr;7(2):136-45. doi: 10.1177/107385840100700208. Neuroscientist. 2001. PMID: 11496924 Review.
Cited by
-
Production of resurgent current in NaV1.6-null Purkinje neurons by slowing sodium channel inactivation with beta-pompilidotoxin.J Neurosci. 2004 Jan 7;24(1):35-42. doi: 10.1523/JNEUROSCI.3807-03.2004. J Neurosci. 2004. PMID: 14715935 Free PMC article.
-
The Hodgkin-Huxley-Katz Prize Lecture: A Markov model with permeation-dependent gating that accounts for resurgent current of voltage-gated Na channels.J Physiol. 2023 Dec;601(23):5147-5164. doi: 10.1113/JP285166. Epub 2023 Oct 14. J Physiol. 2023. PMID: 37837315 Free PMC article.
-
S-Palmitoylation of the sodium channel Nav1.6 regulates its activity and neuronal excitability.J Biol Chem. 2020 May 1;295(18):6151-6164. doi: 10.1074/jbc.RA119.012423. Epub 2020 Mar 11. J Biol Chem. 2020. PMID: 32161114 Free PMC article.
-
Specificity, affinity and efficacy of iota-conotoxin RXIA, an agonist of voltage-gated sodium channels Na(V)1.2, 1.6 and 1.7.Biochem Pharmacol. 2008 Jun 15;75(12):2334-44. doi: 10.1016/j.bcp.2008.03.019. Epub 2008 Apr 6. Biochem Pharmacol. 2008. PMID: 18486102 Free PMC article.
-
Divergent actions of the pyrethroid insecticides S-bioallethrin, tefluthrin, and deltamethrin on rat Na(v)1.6 sodium channels.Toxicol Appl Pharmacol. 2010 Sep 15;247(3):229-37. doi: 10.1016/j.taap.2010.07.001. Epub 2010 Jul 17. Toxicol Appl Pharmacol. 2010. PMID: 20624410 Free PMC article.
References
-
- Auld VJ, Goldin AL, Krafte DS, Marshall J, Dunn JM, Catterall WA, Lester HA, Davidson N, Dunn RJ. A rat brain Na+ channel α subunit with novel gating properties. Neuron. 1988;1:449–461. - PubMed
-
- Black JA, Yokoyama S, Higashida H, Ransom BR, Waxman SG. Sodium channel mRNAs I, II, and III in the CNS: cell-specific expression. Mol Brain Res. 1994;22:275–289. - PubMed
-
- Burgess DL, Kohrman DC, Galt J, Plummer NW, Jones JM, Spear B, Meisler MH. Mutation of a new sodium channel gene, Scn8a, in the mouse mutant “motor endplate disease.”. Nat Genet. 1995;10:461–465. - PubMed
-
- Catterall WA. Cellular and molecular biology of voltage-gated sodium channels. Physiol Rev. 1992;72:S15–S48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
