Migration of mitochondria to viral assembly sites in African swine fever virus-infected cells
- PMID: 9696857
- PMCID: PMC110008
- DOI: 10.1128/JVI.72.9.7583-7588.1998
Migration of mitochondria to viral assembly sites in African swine fever virus-infected cells
Abstract
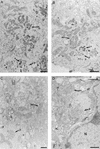
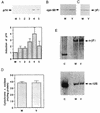
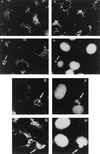
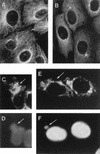
An examination by electron microscopy of the viral assembly sites in Vero cells infected with African swine fever virus showed the presence of large clusters of mitochondria located in their proximity. These clusters surround viral factories that contain assembling particles but not factories where only precursor membranes are seen. Immunofluorescence microscopy revealed that these accumulations of mitochondria are originated by a massive migration of the organelle to the virus assembly sites. Virus infection also promoted the induction of the mitochondrial stress-responsive proteins p74 and cpn 60 together with a dramatic shift in the ultrastructural morphology of the mitochondria toward that characteristic of actively respiring organelles. The clustering of mitochondria around the viral factory was blocked in the presence of the microtubule-disassembling drug nocodazole, indicating that these filaments are implicated in the transport of the mitochondria to the virus assembly sites. The results presented are consistent with a role for the mitochondria in supplying the energy that the virus morphogenetic processes may require and make of the African swine fever virus-infected cell a paradigm to investigate the mechanisms involved in the sorting of mitochondria within the cell.
Figures

Similar articles
-
Transport of African swine fever virus from assembly sites to the plasma membrane is dependent on microtubules and conventional kinesin.J Virol. 2004 Aug;78(15):7990-8001. doi: 10.1128/JVI.78.15.7990-8001.2004. J Virol. 2004. PMID: 15254171 Free PMC article.
-
African swine fever virus infection disrupts centrosome assembly and function.J Gen Virol. 2005 Mar;86(Pt 3):589-594. doi: 10.1099/vir.0.80623-0. J Gen Virol. 2005. PMID: 15722518
-
Unpicking the Secrets of African Swine Fever Viral Replication Sites.Viruses. 2021 Jan 8;13(1):77. doi: 10.3390/v13010077. Viruses. 2021. PMID: 33429879 Free PMC article.
-
Antiviral agents against African swine fever virus.Virus Res. 2019 Sep;270:197669. doi: 10.1016/j.virusres.2019.197669. Epub 2019 Jul 17. Virus Res. 2019. PMID: 31325472 Review.
-
African swine fever virus morphogenesis.Virus Res. 2013 Apr;173(1):29-41. doi: 10.1016/j.virusres.2012.09.016. Epub 2012 Oct 8. Virus Res. 2013. PMID: 23059353 Review.
Cited by
-
Regulation of host translational machinery by African swine fever virus.PLoS Pathog. 2009 Aug;5(8):e1000562. doi: 10.1371/journal.ppat.1000562. Epub 2009 Aug 28. PLoS Pathog. 2009. PMID: 19714237 Free PMC article.
-
African swine fever virus structural protein pE120R is essential for virus transport from assembly sites to plasma membrane but not for infectivity.J Virol. 2001 Aug;75(15):6758-68. doi: 10.1128/JVI.75.15.6758-6768.2001. J Virol. 2001. PMID: 11435554 Free PMC article.
-
Regulation of antiviral immune response by African swine fever virus (ASFV).Virol Sin. 2022 Apr;37(2):157-167. doi: 10.1016/j.virs.2022.03.006. Epub 2022 Mar 9. Virol Sin. 2022. PMID: 35278697 Free PMC article. Review.
-
Dynamics of the mitochondrial reticulum in live cells using Fourier imaging correlation spectroscopy and digital video microscopy.Biophys J. 2000 Oct;79(4):1833-49. doi: 10.1016/S0006-3495(00)76433-2. Biophys J. 2000. PMID: 11023889 Free PMC article.
-
Internal-ribosome-entry-site functional activity of the 3'-untranslated region of the mRNA for the beta subunit of mitochondrial H+-ATP synthase.Biochem J. 2000 Mar 15;346 Pt 3(Pt 3):849-55. Biochem J. 2000. PMID: 10698716 Free PMC article.
References
-
- Alconada A, Flores A I, Blanco L, Cuezva J M. Antibodies against F1-ATPase α-subunit recognize mitochondrial chaperones. Evidence for an evolutionary relationship between chaperonin and ATPase protein families. J Biol Chem. 1994;269:13670–13679. - PubMed
-
- Andrés, G. Personal communication.
-
- Brady S T. A novel brain ATPase with properties expected for the fast axonal transport motor. Nature. 1985;317:73–75. - PubMed
-
- Brady S T, Lasek R J, Allen R D. Fast axonal transport in extruded axoplasm from squid giant axon. Science. 1982;218:1129–1131. - PubMed

