Absence of neurofilaments reduces the selective vulnerability of motor neurons and slows disease caused by a familial amyotrophic lateral sclerosis-linked superoxide dismutase 1 mutant
- PMID: 9689132
- PMCID: PMC21390
- DOI: 10.1073/pnas.95.16.9631
Absence of neurofilaments reduces the selective vulnerability of motor neurons and slows disease caused by a familial amyotrophic lateral sclerosis-linked superoxide dismutase 1 mutant
Abstract
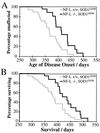
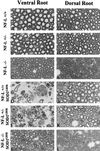
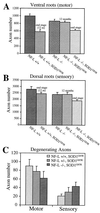
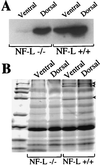
Mutations in superoxide dismutase 1 (SOD1), the only proven cause of amyotrophic lateral sclerosis (ALS), provoke disease through an unidentified toxic property. Neurofilament aggregates are pathologic hallmarks of both sporadic and SOD1-mediated familial ALS. By deleting NF-L, the major neurofilament subunit required for filament assembly, onset and progression of disease caused by familial ALS-linked SOD1 mutant G85R are significantly slowed, while selectivity of mutant-mediated toxicity for motor neurons is reduced. In NF-L-deleted animals, levels of the two remaining neurofilament subunits, NF-M and NF-H, are markedly reduced in axons but are elevated in motor neuron cell bodies. Thus, while neither perikaryal nor axonal neurofilaments are essential for SOD1-mediated disease, the absence of assembled neurofilaments both diminishes selective vulnerability and slows SOD1(G85R) mutant-mediated toxicity to motor neurons.
Figures

Similar articles
-
Altered axonal architecture by removal of the heavily phosphorylated neurofilament tail domains strongly slows superoxide dismutase 1 mutant-mediated ALS.Proc Natl Acad Sci U S A. 2005 Jul 19;102(29):10351-6. doi: 10.1073/pnas.0503862102. Epub 2005 Jul 7. Proc Natl Acad Sci U S A. 2005. PMID: 16002469 Free PMC article.
-
Extra axonal neurofilaments do not exacerbate disease caused by mutant Cu,Zn superoxide dismutase.Neurobiol Dis. 2000 Aug;7(4):462-70. doi: 10.1006/nbdi.2000.0296. Neurobiol Dis. 2000. PMID: 10964615
-
The neuronal Golgi apparatus is fragmented in transgenic mice expressing a mutant human SOD1, but not in mice expressing the human NF-H gene.J Neurol Sci. 2000 Feb 1;173(1):63-72. doi: 10.1016/s0022-510x(99)00301-9. J Neurol Sci. 2000. PMID: 10675581
-
Transgenic mice in the study of ALS: the role of neurofilaments.Brain Pathol. 1998 Oct;8(4):759-69. doi: 10.1111/j.1750-3639.1998.tb00199.x. Brain Pathol. 1998. PMID: 9804382 Free PMC article. Review.
-
Mechanisms of selective motor neuron death in transgenic mouse models of motor neuron disease.Neurology. 1996 Oct;47(4 Suppl 2):S54-61; discussion S61-2. doi: 10.1212/wnl.47.4_suppl_2.54s. Neurology. 1996. PMID: 8858052 Review.
Cited by
-
Complex genetics of amyotrophic lateral sclerosis.Am J Hum Genet. 2004 Dec;75(6):933-47. doi: 10.1086/426001. Epub 2004 Oct 11. Am J Hum Genet. 2004. PMID: 15478096 Free PMC article. Review. No abstract available.
-
Microglia Influence Neurofilament Deposition in ALS iPSC-Derived Motor Neurons.Genes (Basel). 2022 Jan 27;13(2):241. doi: 10.3390/genes13020241. Genes (Basel). 2022. PMID: 35205286 Free PMC article.
-
The usage and advantages of several common amyotrophic lateral sclerosis animal models.Front Neurosci. 2024 Feb 26;18:1341109. doi: 10.3389/fnins.2024.1341109. eCollection 2024. Front Neurosci. 2024. PMID: 38595972 Free PMC article. Review.
-
Peripherin is a subunit of peripheral nerve neurofilaments: implications for differential vulnerability of CNS and peripheral nervous system axons.J Neurosci. 2012 Jun 20;32(25):8501-8. doi: 10.1523/JNEUROSCI.1081-12.2012. J Neurosci. 2012. PMID: 22723690 Free PMC article.
-
Mutation in neurofilament transgene implicates RNA processing in the pathogenesis of neurodegenerative disease.J Neurosci. 1999 Feb 15;19(4):1273-83. doi: 10.1523/JNEUROSCI.19-04-01273.1999. J Neurosci. 1999. PMID: 9952405 Free PMC article.
References
-
- Rosen D R, Siddique T, Patterson D, Figlewicz D A, Sapp P, Hentati A, Donaldson D, Goto J, O’Regan J P, Deng H X, et al. Nature (London) 1993;362:59–62. - PubMed
-
- Deng H-X, Hentati A, Tainer J A, Iqbal Z, Cayabyab A, Hung W-Y, Getzoff E D, Hu P, Herzfeldt B, Roos R P, et al. Science. 1993;261:1047–1051. - PubMed
-
- Siddique, T. & Deng, H. X. (1996) Hum. Mol. Genet. 5, Suppl. 1465–1470. - PubMed
-
- Fridovich I. Annu Rev Biochem. 1995;64:97–112. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

