A role for the actin-bundling protein L-plastin in the regulation of leukocyte integrin function
- PMID: 9689080
- PMCID: PMC21338
- DOI: 10.1073/pnas.95.16.9331
A role for the actin-bundling protein L-plastin in the regulation of leukocyte integrin function
Abstract
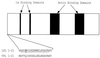
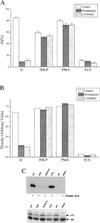
Regulation of leukocyte integrin avidity is a crucial aspect of inflammation and immunity. The actin cytoskeleton has an important role in the regulation of integrin function, but the cytoskeletal proteins involved are largely unknown. Because inflammatory stimuli that activate integrin-mediated adhesion in human polymorphonuclear neutrophils (PMN) and monocytes cause phosphorylation of the actin-bundling protein L-plastin, we tested whether L-plastin phosphorylation was involved in integrin activation. L-plastin-derived peptides that included the phosphorylation site (Ser-5) rapidly induced leukocyte integrin-mediated adhesion when introduced into the cytosol of freshly isolated primary human PMN and monocytes. Substitution of Ala for Ser-5 abolished the ability of the peptide to induce adhesion. Peptide-induced adhesion was sensitive to pharmacologic inhibition of phosphoinositol 3-kinase and protein kinase C, but adhesion induced by a peptide containing a phosphoserine at position 5 was insensitive to inhibition. These data establish a novel role for L-plastin in the regulation of leukocyte adhesion and suggest that many signaling events implicated in integrin regulation act via induction of L-plastin phosphorylation.
Figures
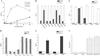

Similar articles
-
Immune complex-induced integrin activation and L-plastin phosphorylation require protein kinase A.J Biol Chem. 1999 Aug 20;274(34):24349-56. doi: 10.1074/jbc.274.34.24349. J Biol Chem. 1999. PMID: 10446213
-
Priming of eosinophils by GM-CSF is mediated by protein kinase CbetaII-phosphorylated L-plastin.J Immunol. 2011 Jun 1;186(11):6485-96. doi: 10.4049/jimmunol.1001868. Epub 2011 Apr 27. J Immunol. 2011. PMID: 21525390 Free PMC article.
-
FcgammaRII-mediated adhesion and phagocytosis induce L-plastin phosphorylation in human neutrophils.J Biol Chem. 1996 Jun 14;271(24):14623-30. doi: 10.1074/jbc.271.24.14623. J Biol Chem. 1996. PMID: 8663066
-
Plastins: versatile modulators of actin organization in (patho)physiological cellular processes.Acta Pharmacol Sin. 2005 Jul;26(7):769-79. doi: 10.1111/j.1745-7254.2005.00145.x. Acta Pharmacol Sin. 2005. PMID: 15960882 Review.
-
The actin-bundling protein L-plastin supports T-cell motility and activation.Immunol Rev. 2013 Nov;256(1):48-62. doi: 10.1111/imr.12102. Immunol Rev. 2013. PMID: 24117812 Free PMC article. Review.
Cited by
-
Actin-bundling protein plastin 3 is a regulator of ectoplasmic specialization dynamics during spermatogenesis in the rat testis.FASEB J. 2015 Sep;29(9):3788-805. doi: 10.1096/fj.14-267997. Epub 2015 Jun 5. FASEB J. 2015. PMID: 26048141 Free PMC article.
-
LFA-1 cluster formation in T-cells depends on L-plastin phosphorylation regulated by P90RSK and PP2A.Cell Mol Life Sci. 2021 Apr;78(7):3543-3564. doi: 10.1007/s00018-020-03744-z. Epub 2021 Jan 15. Cell Mol Life Sci. 2021. PMID: 33449151 Free PMC article.
-
Gene expression versus sequence for predicting function: Glia Maturation Factor gamma is not a glia maturation factor.Genomics Proteomics Bioinformatics. 2003 Feb;1(1):52-7. doi: 10.1016/s1672-0229(03)01007-6. Genomics Proteomics Bioinformatics. 2003. PMID: 15626333 Free PMC article.
-
Dual inhibition of ABCE1 and LCP1 by microRNA-96 results in an additive effect in breast cancer mouse model.Oncotarget. 2019 Mar 12;10(21):2086-2094. doi: 10.18632/oncotarget.26747. eCollection 2019 Mar 12. Oncotarget. 2019. PMID: 31007850 Free PMC article.
-
Metastasis of prostate cancer and melanoma cells in a preclinical in vivo mouse model is enhanced by L-plastin expression and phosphorylation.Mol Cancer. 2014 Jan 18;13:10. doi: 10.1186/1476-4598-13-10. Mol Cancer. 2014. PMID: 24438191 Free PMC article.
References
-
- Berton G, Yan S R, Fumagalli L, Lowell C A. Int J Clin Lab Res. 1996;26:160–177. - PubMed
-
- Diamond M S, Springer T A. Curr Biol. 1994;4:506–517. - PubMed
-
- Wright S D, Meyer B C. J Immunol. 1986;136:1758–1764. - PubMed
-
- Jones S L, Knaus U G, Bokoch G M, Brown E J. J Biol Chem. 1998;273:10556–10566. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

