Kainate induces an intracellular Na+-activated current in cultured embryonic rat hippocampal neurones
- PMID: 9660888
- PMCID: PMC2231087
- DOI: 10.1111/j.1469-7793.1998.721bj.x
Kainate induces an intracellular Na+-activated current in cultured embryonic rat hippocampal neurones
Abstract
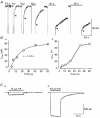
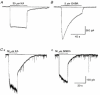
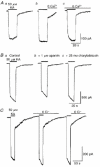
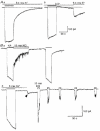
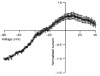
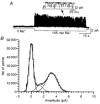
1. In embryonic rat hippocampal neurones cultured for < 3 days, kainate induced an inward current at negative potentials that recovered to baseline levels immediately upon termination of agonist application. However, in neurones cultured for longer, the kainate-induced current was often followed by a long-lasting inward current that slowly recovered to baseline levels. The amplitude of the delayed current (Idelay) triggered by kainate was positively related both to the duration of application at constant agonist concentration and to concentration at constant application duration. 2. Idelay could last for several minutes and was accompanied by a conductance increase, which closely paralleled current amplitude. Depression of the kainate-induced current response at receptor level with CNQX or at ionic level with Na+-free solution eliminated Idelay. However, when applied during Idelay neither CNQX nor Na+-free solution had any effect on Idelay. Li+ effected the same response as Na+ in mediating kainate-induced Idelay. 3. GABA-activated Cl- current, which was associated with the same amount of inwardly directed charge flow at the same potential as that induced by kainate, did not trigger a long-lasting delayed current. 4. Idelay depended on the existence of extracellular K+ and its amplitude increased with the increase in K+ concentration. Neither applying Cl-- or Ca2+-free solutions nor increasing intracellular Ca2+ buffering speed and capacity altered Idelay. Exposure to the specific KCa channel blockers apamin and charybdotoxin also failed to alter Idelay. However, Idelay could be blocked by Cs+, Ba2+ and high concentrations of 4-aminopyridine (4-AP) and TEA. 5. Inside-out excised patch-clamp recordings revealed that low density or highly clustered Na+-activated K+ channels were expressed in the cell bodies of cultured embryonic rat hippocampal neurones. These could be the elementary channels underlying Idelay.
Figures


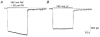

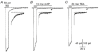
Similar articles
-
Inhibition of delayed rectifier K+ conductance in cultured rat cerebellar granule neurons by activation of calcium-permeable AMPA receptors.Eur J Neurosci. 2000 Mar;12(3):935-44. doi: 10.1046/j.1460-9568.2000.00983.x. Eur J Neurosci. 2000. PMID: 10762323
-
Distribution of neurones expressing inwardly rectifying and Ca(2+)-permeable AMPA receptors in rat hippocampal slices.J Physiol. 1996 Mar 15;491 ( Pt 3)(Pt 3):719-33. doi: 10.1113/jphysiol.1996.sp021252. J Physiol. 1996. PMID: 8815206 Free PMC article.
-
Depression of a sustained calcium current by kainate in rat hippocampal neurones in vitro.J Physiol. 1991 Apr;435:465-81. doi: 10.1113/jphysiol.1991.sp018519. J Physiol. 1991. PMID: 1770444 Free PMC article.
-
AMPA/kainate receptor activation in murine oligodendrocyte precursor cells leads to activation of a cation conductance, calcium influx and blockade of delayed rectifying K+ channels.Neuroscience. 1994 Nov;63(1):135-49. doi: 10.1016/0306-4522(94)90012-4. Neuroscience. 1994. PMID: 7898644
-
Potassium channels activated by sodium.Q J Exp Physiol. 1989 Dec;74(7):1033-41. doi: 10.1113/expphysiol.1989.sp003331. Q J Exp Physiol. 1989. PMID: 2697016 Review.
Cited by
-
Slack and Slick K(Na) channels regulate the accuracy of timing of auditory neurons.J Neurosci. 2007 Mar 7;27(10):2617-27. doi: 10.1523/JNEUROSCI.5308-06.2007. J Neurosci. 2007. PMID: 17344399 Free PMC article.
-
Slack, Slick and Sodium-Activated Potassium Channels.ISRN Neurosci. 2013 Apr 18;2013(2013):354262. doi: 10.1155/2013/354262. ISRN Neurosci. 2013. PMID: 24319675 Free PMC article.
-
Sodium-dependent potassium channels in leech P neurons.J Membr Biol. 2005 Nov;208(1):27-38. doi: 10.1007/s00232-005-0816-x. J Membr Biol. 2005. PMID: 16596444
References
-
- Bader CR, Bernheim L, Bertrand D. Sodium activated potassium current in cultured avian neurons. Nature. 1985;317:540–542. - PubMed
-
- Choi DW. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988;1:623–634. - PubMed
-
- Collingridge GL, Lester RAS. Excitatory amino acid receptors in the vertebrate central nervous system. Pharmacological Reviews. 1989;40:145–195. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

