Apoptosis in feline panleukopenia virus-infected lymphocytes
- PMID: 9658149
- PMCID: PMC109909
- DOI: 10.1128/JVI.72.8.6932-6936.1998
Apoptosis in feline panleukopenia virus-infected lymphocytes
Abstract
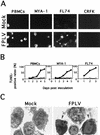
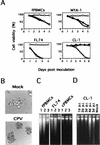
Feline panleukopenia virus (FPLV) was shown to induce apoptosis to feline lymphoid cells and to reduce the expression of interleukin-2 receptor alpha on the cells. FPLV-induced apoptosis might be a key element in the pathophysiology of atrophy of lymphoid tissues associated with feline panleukopenia caused by FPLV.
Figures


Similar articles
-
Panleukopenia-like syndrome of FeLV caused by co-infection with FeLV and feline panleukopenia virus.Vet Immunol Immunopathol. 1995 May;46(1-2):21-33. doi: 10.1016/0165-2427(94)07003-p. Vet Immunol Immunopathol. 1995. PMID: 7618258
-
Characterisation of cross-reactivity of virus neutralising antibodies induced by feline panleukopenia virus and canine parvoviruses.Res Vet Sci. 2001 Dec;71(3):219-22. doi: 10.1053/rvsc.2001.0492. Res Vet Sci. 2001. PMID: 11798298
-
Regional adaptations and parallel mutations in Feline panleukopenia virus strains from China revealed by nearly-full length genome analysis.PLoS One. 2020 Jan 16;15(1):e0227705. doi: 10.1371/journal.pone.0227705. eCollection 2020. PLoS One. 2020. PMID: 31945103 Free PMC article.
-
Feline parvovirus infection and associated diseases.Vet J. 2014 Aug;201(2):150-5. doi: 10.1016/j.tvjl.2014.05.027. Epub 2014 May 22. Vet J. 2014. PMID: 24923754 Review.
-
Pathogenesis of feline panleukopenia virus and canine parvovirus.Baillieres Clin Haematol. 1995 Mar;8(1):57-71. doi: 10.1016/s0950-3536(05)80232-x. Baillieres Clin Haematol. 1995. PMID: 7663051 Free PMC article. Review.
Cited by
-
Human Parvovirus Infection of Human Airway Epithelia Induces Pyroptotic Cell Death by Inhibiting Apoptosis.J Virol. 2017 Nov 30;91(24):e01533-17. doi: 10.1128/JVI.01533-17. Print 2017 Dec 15. J Virol. 2017. PMID: 29021400 Free PMC article.
-
Nuclear envelope disruption involving host caspases plays a role in the parvovirus replication cycle.J Virol. 2011 May;85(10):4863-74. doi: 10.1128/JVI.01999-10. Epub 2011 Mar 2. J Virol. 2011. PMID: 21367902 Free PMC article.
-
True versus false parasite interactions: a robust method to take risk factors into account and its application to feline viruses.PLoS One. 2012;7(1):e29618. doi: 10.1371/journal.pone.0029618. Epub 2012 Jan 3. PLoS One. 2012. PMID: 22235312 Free PMC article.
-
Feline host range of canine parvovirus: recent emergence of new antigenic types in cats.Emerg Infect Dis. 2002 Apr;8(4):341-6. doi: 10.3201/eid0804.010228. Emerg Infect Dis. 2002. PMID: 11971764 Free PMC article.
-
Apoptosis in murine norovirus-infected RAW264.7 cells is associated with downregulation of survivin.J Virol. 2009 Apr;83(8):3647-56. doi: 10.1128/JVI.02028-08. Epub 2009 Feb 11. J Virol. 2009. PMID: 19211757 Free PMC article.
References
-
- Ackley C D, Cooper M D. Characterization of a feline T-cell-specific monoclonal antibody reactive with a CD5-like molecule. Am J Vet Res. 1992;53:466–471. - PubMed
-
- Azetaka M, Hirasawa T, Konishi S, Ogata M. Studies on canine parvovirus isolation, experimental infection and serologic survey. Jpn J Vet Sci. 1981;43:243–255. - PubMed
-
- Crandell R A, Fabricant C G, Nelson-Rees W A. Development, characterization and viral susceptibility of a feline (Felis catus) renal cell line (CRFK) In Vitro (Rockville) 1973;9:176–185. - PubMed
-
- Goto H, Hirano T, Uchida E, Watanabe K, Shinagawa M, Ichijo S, Shimizu K. Comparative studies of physicochemical and biological properties between canine parvovirus and feline panleukopenia virus. Jpn J Vet Sci. 1984;46:519–526. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

