Steady-state plasma membrane expression of human cytomegalovirus gB is determined by the phosphorylation state of Ser900
- PMID: 9658112
- PMCID: PMC109858
- DOI: 10.1128/JVI.72.8.6657-6664.1998
Steady-state plasma membrane expression of human cytomegalovirus gB is determined by the phosphorylation state of Ser900
Abstract
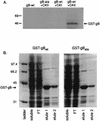
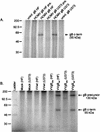
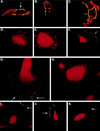
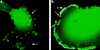
Human cytomegalovirus (HCMV) infection of an astrocytoma cell line (U373) or human fibroblast (HF) cells results in a differential cell distribution of the major envelope glycoprotein gB (UL55). This 906-amino-acid type I glycoprotein contains an extracellular domain with a signal sequence, a transmembrane domain, and a 135-amino-acid cytoplasmic tail with a consensus casein kinase II (CKII) site located at Ser900. Since phosphorylation of proteins in the secretory pathway is an important determinant of intracellular trafficking, the state of gB phosphorylation in U373 and HF cells was examined. Analysis of cells expressing wild-type gB and gB with site-specific mutations indicated that the glycoprotein was equally phosphorylated at a single site, Ser900, in both U373 and HF cells. To assess the effect of charge on gB surface expression in U373 cells, Ser900 was replaced with an aspartate (Asp) or alanine (Ala) residue to mimic the phosphorylated and nonphosphorylated states, respectively. Expression of the Asp but not the Ala gB mutation resulted in an increase in the steady-state expression of gB at the plasma membrane (PM) in U373 cells. In addition, treatment of U373 cells with the phosphatase inhibitor tautomycin resulted in the accumulation of gB at the PM. Interestingly, the addition of a charge at Ser900 trapped gB in a low-level cycling pathway at the PM, preventing trafficking of the protein to the trans-Golgi network or other intracellular compartments. Therefore, these results suggest that a tautomycin-sensitive phosphatase regulates cell-specific PM retrieval of gB to intracellular compartments.
Figures


Similar articles
-
Retrieval of human cytomegalovirus glycoprotein B from cell surface is not required for virus envelopment in astrocytoma cells.J Virol. 2002 May;76(10):5147-55. doi: 10.1128/jvi.76.10.5147-5155.2002. J Virol. 2002. PMID: 11967330 Free PMC article.
-
Phosphorylation of human cytomegalovirus glycoprotein B (gB) at the acidic cluster casein kinase 2 site (Ser900) is required for localization of gB to the trans-Golgi network and efficient virus replication.J Virol. 2004 Jan;78(1):285-93. doi: 10.1128/jvi.78.1.285-293.2004. J Virol. 2004. PMID: 14671110 Free PMC article.
-
Mutated forms of human cytomegalovirus glycoprotein B are impaired in inducing syncytium formation.Virology. 1995 Jun 1;209(2):580-91. doi: 10.1006/viro.1995.1290. Virology. 1995. PMID: 7778290
-
Synthesis of specific protein phosphatase inhibitors, tautomycin and tautomycetin toward structure-activity relationship study.Curr Med Chem. 2002 Nov;9(22):2033-53. doi: 10.2174/0929867023368818. Curr Med Chem. 2002. PMID: 12369869 Review.
-
A Native Human Monoclonal Antibody Targeting HCMV gB (AD-2 Site I).Int J Mol Sci. 2018 Dec 11;19(12):3982. doi: 10.3390/ijms19123982. Int J Mol Sci. 2018. PMID: 30544903 Free PMC article. Review.
Cited by
-
Retrieval of human cytomegalovirus glycoprotein B from cell surface is not required for virus envelopment in astrocytoma cells.J Virol. 2002 May;76(10):5147-55. doi: 10.1128/jvi.76.10.5147-5155.2002. J Virol. 2002. PMID: 11967330 Free PMC article.
-
Intracellular formation and processing of the heterotrimeric gH-gL-gO (gCIII) glycoprotein envelope complex of human cytomegalovirus.J Virol. 1999 May;73(5):3886-92. doi: 10.1128/JVI.73.5.3886-3892.1999. J Virol. 1999. PMID: 10196283 Free PMC article.
-
Phosphorylation and dephosphorylation of calsequestrin on CK2-sensitive sites in heart.Mol Cell Biochem. 2004 Nov;266(1-2):209-17. doi: 10.1023/b:mcbi.0000049164.28580.56. Mol Cell Biochem. 2004. PMID: 15646044
-
Trafficking to the plasma membrane of the seven-transmembrane protein encoded by human herpesvirus 6 U51 gene involves a cell-specific function present in T lymphocytes.J Virol. 1999 Jan;73(1):325-33. doi: 10.1128/JVI.73.1.325-333.1999. J Virol. 1999. PMID: 9847336 Free PMC article.
-
The extracellular domain of herpes simplex virus gE is sufficient for accumulation at cell junctions but not for cell-to-cell spread.J Virol. 2000 Mar;74(5):2278-87. doi: 10.1128/jvi.74.5.2278-2287.2000. J Virol. 2000. PMID: 10666258 Free PMC article.
References
-
- Bogner E, Anheier B, Offner F, Smuda C, Reschke M, Eickmann M, Radsak K. Nuclear translocation of mutagenized forms of human cytomegalovirus glycoprotein B (gpUL55) J Gen Virol. 1997;78:1647–1651. - PubMed
-
- Bogner E, Reschke M, Reis B, Reis E, Britt W, Radsak K. Recognition of compartmentalized intracellular analogs of glycoprotein H of human cytomegalovirus. Arch Virol. 1992;126:67–80. - PubMed
-
- Britt W J. Neutralizing antibodies detect a disulfide-linked glycoprotein complex within the envelope of human cytomegalovirus. Virology. 1984;135:369–378. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

