Binding of human immunodeficiency virus type 1 to CD4 and CXCR4 receptors differentially regulates expression of inflammatory genes and activates the MEK/ERK signaling pathway
- PMID: 9658081
- PMCID: PMC109793
- DOI: 10.1128/JVI.72.8.6406-6413.1998
Binding of human immunodeficiency virus type 1 to CD4 and CXCR4 receptors differentially regulates expression of inflammatory genes and activates the MEK/ERK signaling pathway
Abstract
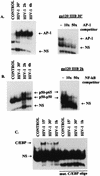
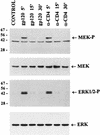
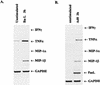
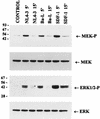
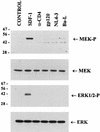
We have previously shown that binding of human immunodeficiency virus type 1 (HIV-1) virions to CD4 receptors stimulates association of Lck with Raf-1 and results in the activation of Raf-1 kinase in a Ras-independent manner. In the present study, we demonstrate that HIV-1 envelope glycoproteins of both T-cell-tropic and macrophagetropic strains rapidly activate the ERK/mitogen-activated protein (MAP) kinase pathway and the binding of nuclear transcription factors (AP-1, NF-kappaB, and C/EBP) and stimulate expression of cytokine and chemokine genes. The activation of this signaling pathway requires functional CD4 receptors and is independent of binding to CXCR4. Binding of the natural ligand stromal cell-derived factor 1 (SDF-1) to CXCR4, which inhibits entry of T-cell-tropic HIV-1, activates also the ERK/MAP kinase pathway. However, SDF-1 did not affect the CD4-mediated expression of cytokine and chemokine genes. These results provide firm molecular evidence that binding of HIV-1 envelope glycoproteins to CD4 receptor initiates a signaling pathway(s) independent of the binding to the chemokine receptor that leads to the aberrant expression of inflammatory genes and may contribute significantly to HIV-1 replication as well as to deregulation of the immune system.
Figures
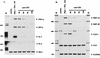


Similar articles
-
Involvement of extracellular signal-regulated kinase module in HIV-mediated CD4 signals controlling activation of nuclear factor-kappa B and AP-1 transcription factors.J Immunol. 1998 Feb 15;160(4):1875-85. J Immunol. 1998. PMID: 9469449
-
Early activation of mitogen-activated protein kinase kinase, extracellular signal-regulated kinase, p38 mitogen-activated protein kinase, and c-Jun N-terminal kinase in response to binding of simian immunodeficiency virus to Jurkat T cells expressing CCR5 receptor.Virology. 1998 Dec 5;252(1):210-7. doi: 10.1006/viro.1998.9466. Virology. 1998. PMID: 9875330
-
HIV-1 glycoprotein 120 induces the MMP-9 cytopathogenic factor production that is abolished by inhibition of the p38 mitogen-activated protein kinase signaling pathway.Blood. 2001 Aug 1;98(3):541-7. doi: 10.1182/blood.v98.3.541. Blood. 2001. PMID: 11468147
-
Chemokine receptor utilization and macrophage signaling by human immunodeficiency virus type 1 gp120: Implications for neuropathogenesis.J Neurovirol. 2004;10 Suppl 1:91-6. doi: 10.1080/753312758. J Neurovirol. 2004. PMID: 14982745 Review.
-
Signaling through the P38 and ERK pathways: a common link between HIV replication and the immune response.Immunol Res. 2010 Dec;48(1-3):99-109. doi: 10.1007/s12026-010-8170-1. Immunol Res. 2010. PMID: 20725863 Review.
Cited by
-
Phosphorylation of PI3K/Akt and MAPK/ERK in an early entry step of enterovirus 71.Life Sci. 2005 Nov 19;78(1):82-90. doi: 10.1016/j.lfs.2005.04.076. Epub 2005 Sep 8. Life Sci. 2005. PMID: 16150462 Free PMC article.
-
Human immunodeficiency virus type 1 enters brain microvascular endothelia by macropinocytosis dependent on lipid rafts and the mitogen-activated protein kinase signaling pathway.J Virol. 2002 Jul;76(13):6689-700. doi: 10.1128/jvi.76.13.6689-6700.2002. J Virol. 2002. PMID: 12050382 Free PMC article.
-
[Molecular biology approach to explain lymphatic metastasis].Urologe A. 2005 Jun;44(6):608-13. doi: 10.1007/s00120-005-0833-5. Urologe A. 2005. PMID: 15912324 Review. German.
-
MEK-specific inhibitor U0126 blocks spread of Borna disease virus in cultured cells.J Virol. 2001 May;75(10):4871-7. doi: 10.1128/JVI.75.10.4871-4877.2001. J Virol. 2001. PMID: 11312358 Free PMC article.
-
Swine acute diarrhea syndrome coronavirus replication is reduced by inhibition of the extracellular signal-regulated kinase (ERK) signaling pathway.Virology. 2022 Jan 2;565:96-105. doi: 10.1016/j.virol.2021.10.009. Epub 2021 Oct 30. Virology. 2022. PMID: 34768113 Free PMC article.
References
-
- Amara A, Gall S L, Schwartz O, Salamero J, Montes M, Loetscher P, Baggiolini M, Virelizier J L, Arenzana-Seisdedos F. HIV coreceptor downregulation as antiviral principle: SDF-1 alpha-dependent internalization of the chemokine receptor CXCR4 contributes to inhibition of HIV replication. J Exp Med. 1997;186:139–146. - PMC - PubMed
-
- Arenzana-Seisdedos F, Virelizier J L, Rousset D, Clark-Lewis I, Loetscher P, Moser B, Baggiolini M. HIV blocked by chemokine antagonist. Nature. 1996;383:400. - PubMed
-
- Atchison R E, Gosling J, Monteclaro F S, Franci C, Digilio L, Charo I F, Goldsmith M A. Multiple extracellular elements of CCR5 and HIV-1 entry: dissociation from response to chemokines. Science. 1996;274:1924–1926. - PubMed
-
- Benkirane M, Schmid-Antomarchi H, Littman D R, Hirn M, Rossi B, Devaux C. The cytoplasmic tail of CD4 is required for inhibition of human immunodeficiency virus type 1 replication by antibodies that bind to the immunoglobulin CDR3-like region in domain 1 of CD4. J Virol. 1995;69:6904–6910. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

