Supramolecular organization of immature and mature murine leukemia virus revealed by electron cryo-microscopy: implications for retroviral assembly mechanisms
- PMID: 9636143
- PMCID: PMC22596
- DOI: 10.1073/pnas.95.13.7299
Supramolecular organization of immature and mature murine leukemia virus revealed by electron cryo-microscopy: implications for retroviral assembly mechanisms
Abstract
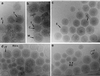
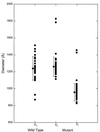
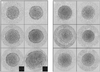
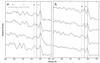
We have used electron cryo-microscopy and image analysis to examine the native structure of immature, protease-deficient (PR-) and mature, wild-type (WT) Moloney murine leukemia virus (MuLV). Maturational cleavage of the Gag polyprotein by the viral protease is associated with striking morphological changes. The PR- MuLV particles exhibit a rounded central core, which has a characteristic track-like shell on its surface, whereas the WT MuLV cores display a polygonal surface with loss of the track-like feature. The pleomorphic shape and inability to refine unique orientation angles suggest that neither the PR- nor the WT MuLV adheres to strict icosahedral symmetry. Nevertheless, the PR- MuLV particles do exhibit paracrystalline order with a spacing between Gag molecules of approximately 45 A and a length of approximately 200 A. Because of the pleomorphic shape and paracrystalline packing of the Gag-RNA complexes, we raise the possibility that assembly of MuLV is driven by protein-RNA, as well as protein-protein, interactions. The maturation process involves a dramatic reorganization of the packing arrangements within the ribonucleoprotein core with disordering and loosening of the individual protein components.
Figures

Similar articles
-
Consideration of the three-dimensional structure of core shells (capsids) in spherical retroviruses.Micron. 2007;38(5):462-70. doi: 10.1016/j.micron.2006.11.007. Epub 2006 Dec 19. Micron. 2007. PMID: 17223564
-
Murine leukemia virus maturation: protease region required for conversion from "immature" to "mature" core form and for virus infectivity.Virology. 1985 Sep;145(2):280-92. doi: 10.1016/0042-6822(85)90161-8. Virology. 1985. PMID: 2411050
-
Assembly and composition of intracellular particles formed by Moloney murine leukemia virus.J Virol. 1993 Sep;67(9):5163-74. doi: 10.1128/JVI.67.9.5163-5174.1993. J Virol. 1993. PMID: 8350394 Free PMC article.
-
Moloney murine leukemia virus protease expressed in bacteria is enzymatically active.Arch Virol. 1998;143(2):381-8. doi: 10.1007/s007050050294. Arch Virol. 1998. PMID: 9541621
-
Noninfectious virus-like particles produced by Moloney murine leukemia virus-based retrovirus packaging cells deficient in viral envelope become infectious in the presence of lipofection reagents.Proc Natl Acad Sci U S A. 1997 Sep 30;94(20):10803-8. doi: 10.1073/pnas.94.20.10803. Proc Natl Acad Sci U S A. 1997. PMID: 9380714 Free PMC article.
Cited by
-
Neutral sphingomyelinase 2 is required for HIV-1 maturation.Proc Natl Acad Sci U S A. 2023 Jul 11;120(28):e2219475120. doi: 10.1073/pnas.2219475120. Epub 2023 Jul 5. Proc Natl Acad Sci U S A. 2023. PMID: 37406093 Free PMC article.
-
HIV-2 Immature Particle Morphology Provides Insights into Gag Lattice Stability and Virus Maturation.J Mol Biol. 2023 Aug 1;435(15):168143. doi: 10.1016/j.jmb.2023.168143. Epub 2023 May 6. J Mol Biol. 2023. PMID: 37150290 Free PMC article.
-
An immunogold single extracellular vesicular RNA and protein (Au SERP) biochip to predict responses to immunotherapy in non-small cell lung cancer patients.J Extracell Vesicles. 2022 Sep;11(9):e12258. doi: 10.1002/jev2.12258. J Extracell Vesicles. 2022. PMID: 36093740 Free PMC article.
-
Molecular Biology and Diversification of Human Retroviruses.Front Virol. 2022;2:872599. doi: 10.3389/fviro.2022.872599. Epub 2022 Jun 2. Front Virol. 2022. PMID: 35783361 Free PMC article.
-
Unique Aggregation of Retroviral Particles Pseudotyped with the Delta Variant SARS-CoV-2 Spike Protein.Viruses. 2022 May 11;14(5):1024. doi: 10.3390/v14051024. Viruses. 2022. PMID: 35632764 Free PMC article.
References
-
- Varmus H, Brown P. In: Mobile DNA. Howe M M, Berg D E, editors. Washington, DC: Am. Soc. Microbiol.; 1989. pp. 53–108.
-
- Coffin J M. In: Fields Virology. Fields B N, Knipe D M, Howley P M, et al., editors. Philadelphia: Lippincott; 1996. pp. 1767–1847.
-
- Ringe D. Methods Enzymol. 1994;241:157–177. - PubMed
-
- Kohlstaedt L A, Wang J, Friedman J M, Rice P A, Steitz T A. Science. 1992;256:1783–1790. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

