Evidence for metabotropic glutamate receptor activation in the induction of depolarization-induced suppression of inhibition in hippocampal CA1
- PMID: 9634553
- PMCID: PMC6792551
- DOI: 10.1523/JNEUROSCI.18-13-04870.1998
Evidence for metabotropic glutamate receptor activation in the induction of depolarization-induced suppression of inhibition in hippocampal CA1
Abstract
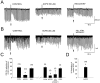
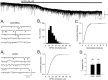
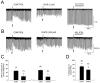
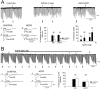
Depolarization-induced suppression of inhibition (DSI) is a transient reduction of GABAA receptor-mediated IPSCs that is mediated by a retrograde signal from principal cells to interneurons. Using whole-cell recordings, we tested the hypothesis that mGluRs are involved in the DSI process in hippocampal CA1, as has been proposed for cerebellar DSI. Group II mGluR agonists failed to affect either evoked monosynaptic IPSCs or DSI, and forskolin, which blocks cerebellar DSI, did not affect CA1 DSI. Group I and group III mGluR agonists reduced IPSCs, but only group I agonists occluded DSI. (S)-MCPG blocked (1S,3R)-ACPD-induced IPSC suppression and markedly reduced DSI, whereas group III antagonists had no effect on DSI. Many other similarities between DSI and the (1S,3R)-ACPD-induced suppression of IPSCs also were found. Our data suggest that a glutamate-like substance released from pyramidal cells could mediate CA1 DSI by reducing GABA release from interneurons via the activation of group I mGluRs.
Figures




Similar articles
-
Differential effects of the group II mGluR agonist, DCG-IV, on depolarization-induced suppression of inhibition in hippocampal CA1 and CA3 neurons.Hippocampus. 2000;10(3):261-8. doi: 10.1002/1098-1063(2000)10:3<261::AID-HIPO6>3.0.CO;2-1. Hippocampus. 2000. PMID: 10902895
-
Heterologous modulation of inhibitory synaptic transmission by metabotropic glutamate receptors in cultured hippocampal neurons.J Neurophysiol. 1996 Feb;75(2):885-93. doi: 10.1152/jn.1996.75.2.885. J Neurophysiol. 1996. PMID: 8714661
-
Activation of group I mGluRs increases spontaneous IPSC frequency in rat frontal cortex.J Neurophysiol. 1998 Aug;80(2):621-7. doi: 10.1152/jn.1998.80.2.621. J Neurophysiol. 1998. PMID: 9705455
-
Pharmacology of postsynaptic metabotropic glutamate receptors in rat hippocampal CA1 pyramidal neurones.Br J Pharmacol. 1995 Sep;116(2):1859-69. doi: 10.1111/j.1476-5381.1995.tb16674.x. Br J Pharmacol. 1995. PMID: 8528571 Free PMC article.
-
Evidence for endogenous excitatory amino acids as mediators in DSI of GABA(A)ergic transmission in hippocampal CA1.J Neurophysiol. 1999 Nov;82(5):2556-64. doi: 10.1152/jn.1999.82.5.2556. J Neurophysiol. 1999. PMID: 10561426
Cited by
-
Endocannabinoid-mediated short-term synaptic plasticity: depolarization-induced suppression of inhibition (DSI) and depolarization-induced suppression of excitation (DSE).Br J Pharmacol. 2004 May;142(1):9-19. doi: 10.1038/sj.bjp.0705726. Epub 2004 Apr 20. Br J Pharmacol. 2004. PMID: 15100161 Free PMC article. Review.
-
Colocalization of FM1-43, Bassoon, and GnRH-1: GnRH-1 release from cell bodies and their neuroprocesses.Endocrinology. 2011 Nov;152(11):4310-21. doi: 10.1210/en.2011-1416. Epub 2011 Sep 6. Endocrinology. 2011. PMID: 21896672 Free PMC article.
-
Optogenetic identification of an intrinsic cholinergically driven inhibitory oscillator sensitive to cannabinoids and opioids in hippocampal CA1.J Physiol. 2014 Jan 1;592(1):103-23. doi: 10.1113/jphysiol.2013.257428. Epub 2013 Nov 4. J Physiol. 2014. PMID: 24190932 Free PMC article.
-
Alterations in metabotropic glutamate receptor 1α and regulator of G protein signaling 4 in the prefrontal cortex in schizophrenia.Am J Psychiatry. 2010 Dec;167(12):1489-98. doi: 10.1176/appi.ajp.2010.10030318. Epub 2010 Oct 1. Am J Psychiatry. 2010. PMID: 20889653 Free PMC article.
-
Presynaptic mGlu1 Receptors Control GABAB Receptors in an Antagonist-Like Manner in Mouse Cortical GABAergic and Glutamatergic Nerve Endings.Front Mol Neurosci. 2018 Sep 18;11:324. doi: 10.3389/fnmol.2018.00324. eCollection 2018. Front Mol Neurosci. 2018. PMID: 30279647 Free PMC article.
References
-
- Alger BE, Pitler TA. Retrograde signaling at GABAA receptor synapses in the mammalian CNS. Trends Neurosci. 1995;18:333–340. - PubMed
-
- Alger BE, Morishita W, Kirov SA. Evaluation of glutamate as the retrograde signal in hippocampal CA1 DSI. Soc Neurosci Abstr. 1997;23:10.
-
- Arague A, Haydon PG. Astrocytes modulate evoked synaptic transmission between cultured hippocampal neurons. Soc Neurosci Abstr. 1997;23:359.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
