Tyrosine 1101 of Tie2 is the major site of association of p85 and is required for activation of phosphatidylinositol 3-kinase and Akt
- PMID: 9632797
- PMCID: PMC108997
- DOI: 10.1128/MCB.18.7.4131
Tyrosine 1101 of Tie2 is the major site of association of p85 and is required for activation of phosphatidylinositol 3-kinase and Akt
Abstract
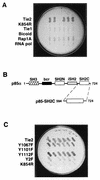
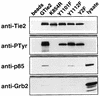
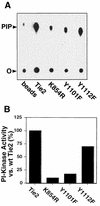
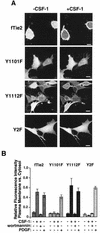
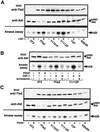
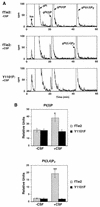
Tie2 is an endothelium-specific receptor tyrosine kinase that is required for both normal embryonic vascular development and tumor angiogenesis and is thought to play a role in vascular maintenance. However, the signaling pathways responsible for the function of Tie2 remain unknown. In this report, we demonstrate that the p85 subunit of phosphatidylinositol 3-kinase (PI3-kinase) associates with Tie2 and that this association confers functional lipid kinase activity. Mutation of tyrosine 1101 of Tie2 abrogated p85 association both in vitro and in vivo in yeast. Tie2 was found to activate PI3-kinase in vivo as demonstrated by direct measurement of increases in cellular phosphatidylinositol 3-phosphate and phosphatidylinositol 3, 4-bisphosphate, by plasma membrane translocation of a green fluorescent protein-Akt pleckstrin homology domain fusion protein, and by downstream activation of the Akt kinase. Activation of PI3-kinase was abrogated in these assays by mutation of Y1101 to phenylalanine, consistent with a requirement for this residue for p85 association with Tie2. These results suggest that activation of PI3-kinase and Akt may in part account for Tie2's role in both embryonic vascular development and pathologic angiogenesis, and they are consistent with a role for Tie2 in endothelial cell survival.
Figures



Similar articles
-
The endothelial receptor tyrosine kinase Tie1 activates phosphatidylinositol 3-kinase and Akt to inhibit apoptosis.Mol Cell Biol. 2002 Mar;22(6):1704-13. doi: 10.1128/MCB.22.6.1704-1713.2002. Mol Cell Biol. 2002. PMID: 11865050 Free PMC article.
-
Stimulation of phosphatidylinositol 3-kinase by fibroblast growth factor receptors is mediated by coordinated recruitment of multiple docking proteins.Proc Natl Acad Sci U S A. 2001 May 22;98(11):6074-9. doi: 10.1073/pnas.111114298. Epub 2001 May 15. Proc Natl Acad Sci U S A. 2001. PMID: 11353842 Free PMC article.
-
The insulin receptor substrate (IRS)-1 recruits phosphatidylinositol 3-kinase to Ret: evidence for a competition between Shc and IRS-1 for the binding to Ret.Oncogene. 2001 Jan 11;20(2):209-18. doi: 10.1038/sj.onc.1204049. Oncogene. 2001. PMID: 11313948
-
Src kinase mediates phosphatidylinositol 3-kinase/Akt-dependent rapid endothelial nitric-oxide synthase activation by estrogen.J Biol Chem. 2003 Jan 24;278(4):2118-23. doi: 10.1074/jbc.M210828200. Epub 2002 Nov 12. J Biol Chem. 2003. PMID: 12431978
-
Functional significance of Tie2 signaling in the adult vasculature.Recent Prog Horm Res. 2004;59:51-71. doi: 10.1210/rp.59.1.51. Recent Prog Horm Res. 2004. PMID: 14749497 Review.
Cited by
-
An agonistic anti-Tie2 antibody suppresses the normal-to-tumor vascular transition in the glioblastoma invasion zone.Exp Mol Med. 2023 Feb;55(2):470-484. doi: 10.1038/s12276-023-00939-9. Epub 2023 Feb 24. Exp Mol Med. 2023. PMID: 36828931 Free PMC article.
-
The endothelial receptor tyrosine kinase Tie1 activates phosphatidylinositol 3-kinase and Akt to inhibit apoptosis.Mol Cell Biol. 2002 Mar;22(6):1704-13. doi: 10.1128/MCB.22.6.1704-1713.2002. Mol Cell Biol. 2002. PMID: 11865050 Free PMC article.
-
Small-molecule inhibitors of the PI3K signaling network.Future Med Chem. 2011 Apr;3(5):549-65. doi: 10.4155/fmc.11.12. Future Med Chem. 2011. PMID: 21526896 Free PMC article. Review.
-
Targeting Protein Kinases to Enhance the Response to anti-PD-1/PD-L1 Immunotherapy.Int J Mol Sci. 2019 May 9;20(9):2296. doi: 10.3390/ijms20092296. Int J Mol Sci. 2019. PMID: 31075880 Free PMC article. Review.
-
Polyphosphoinositide binding domains: Key to inositol lipid biology.Biochim Biophys Acta. 2015 Jun;1851(6):746-58. doi: 10.1016/j.bbalip.2015.02.013. Epub 2015 Feb 27. Biochim Biophys Acta. 2015. PMID: 25732852 Free PMC article. Review.
References
-
- Bae Y S, Cantley L G, Chen C-S, Kim S-R, Kwon K-S, Rhee S G. Activation of phospholipase C-γ by phosphatidylinositol 3,4,5-triphosphate. J Biol Chem. 1998;273:4465–4469. - PubMed
-
- Carpenter C L, Cantley L C. Phosphoinositide kinases. Curr Opin Cell Biol. 1996;8:153–158. - PubMed
-
- Chen H-C, Appeddu P A, Isoda H, Guan J-L. Phosphorylation of tyrosine 397 in focal adhesion kinase is required for binding phosphatidylinositol 3-kinase. J Biol Chem. 1996;271:26329–26334. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
