Potential role of epithelial cell-derived histone H1 proteins in innate antimicrobial defense in the human gastrointestinal tract
- PMID: 9632593
- PMCID: PMC108340
- DOI: 10.1128/IAI.66.7.3255-3263.1998
Potential role of epithelial cell-derived histone H1 proteins in innate antimicrobial defense in the human gastrointestinal tract
Abstract
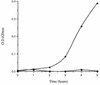
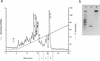
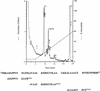
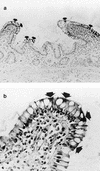
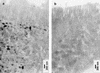
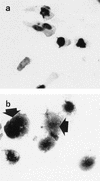
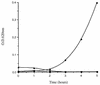
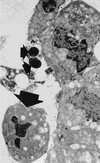
In the human gastrointestinal tract, microorganisms are present in large numbers in the colon but are sparse in the proximal small intestine. In this study, we have shown that acid extracts of fresh human terminal ileal mucosal samples mediate antimicrobial activity. Following cation-exchange chromatography, one of the eluted fractions demonstrated antibacterial activity against bacteria normally resident in the human colonic lumen. This activity was further fractionated by reverse-phase high-performance liquid chromatography and identified as histone H1 and its fragments. We have also shown that in tissue sections, immunoreactive histone H1 is present in the cytoplasm of villus epithelial cells. In vitro culturing of detached (from the basement membrane) villus epithelial cells led to the release of antimicrobial histone H1 proteins, while the cells demonstrated ultrastructural features of programmed cell death. Our studies suggest that cytoplasmic histone H1 may provide protection against penetration by microorganisms into villus epithelial cells. Moreover, intestinal epithelial cells released into the lumen may mediate antimicrobial activity by releasing histone H1 proteins and their fragments.
Figures


Similar articles
-
Alpha-defensins in the gastrointestinal tract.Mol Immunol. 2003 Nov;40(7):463-7. doi: 10.1016/s0161-5890(03)00157-3. Mol Immunol. 2003. PMID: 14568393 Review.
-
Antimicrobial polypeptides of the human colonic epithelium.Peptides. 2003 Nov;24(11):1763-70. doi: 10.1016/j.peptides.2003.07.028. Peptides. 2003. PMID: 15019208
-
Histone H1 proteins act as receptors for the 987P fimbriae of enterotoxigenic Escherichia coli.J Biol Chem. 2005 Jun 17;280(24):23057-65. doi: 10.1074/jbc.M503676200. Epub 2005 Apr 19. J Biol Chem. 2005. PMID: 15840569
-
Development, validation and implementation of an in vitro model for the study of metabolic and immune function in normal and inflamed human colonic epithelium.Dan Med J. 2015 Jan;62(1):B4973. Dan Med J. 2015. PMID: 25557335 Review.
-
The microheterogeneity of the mammalian H1(0) histone. Evidence for an age-dependent deamidation.J Biol Chem. 1998 May 22;273(21):13324-30. doi: 10.1074/jbc.273.21.13324. J Biol Chem. 1998. PMID: 9582379
Cited by
-
Topical antimicrobials for burn wound infections.Recent Pat Antiinfect Drug Discov. 2010 Jun;5(2):124-51. doi: 10.2174/157489110791233522. Recent Pat Antiinfect Drug Discov. 2010. PMID: 20429870 Free PMC article. Review.
-
Linker Histone in Diseases.Int J Biol Sci. 2017 Jul 18;13(8):1008-1018. doi: 10.7150/ijbs.19891. eCollection 2017. Int J Biol Sci. 2017. PMID: 28924382 Free PMC article. Review.
-
The Protective Effect against Extracellular Histones Afforded by Long-Pentraxin PTX3 as a Regulator of NETs.Front Immunol. 2016 Sep 7;7:344. doi: 10.3389/fimmu.2016.00344. eCollection 2016. Front Immunol. 2016. PMID: 27656184 Free PMC article. Review.
-
Acipensins - Novel Antimicrobial Peptides from Leukocytes of the Russian Sturgeon Acipenser gueldenstaedtii.Acta Naturae. 2014 Oct;6(4):99-109. Acta Naturae. 2014. PMID: 25558400 Free PMC article.
-
Histone H4 is a major component of the antimicrobial action of human sebocytes.J Invest Dermatol. 2009 Oct;129(10):2489-96. doi: 10.1038/jid.2009.106. Epub 2009 Jun 18. J Invest Dermatol. 2009. PMID: 19536143 Free PMC article.
References
-
- Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. - PubMed
-
- Edman P. Method of determination of the amino acid sequence in peptides. Acta Chem Scand. 1950;4:283–293.
-
- Erlandsen S L, Parsons J A, Taylor T D. Ultrastructural immunocytochemical localization of lysozyme in the Paneth cells of man. J Histochem Cytochem. 1974;22:401–413. - PubMed
-
- Fields P I, Groisman E A, Heffron F. A Salmonella locus that controls resistance to microbicidal proteins from phagocytic cells. Science. 1989;243:1059–1062. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

