The human toll signaling pathway: divergence of nuclear factor kappaB and JNK/SAPK activation upstream of tumor necrosis factor receptor-associated factor 6 (TRAF6)
- PMID: 9625770
- PMCID: PMC2212359
- DOI: 10.1084/jem.187.12.2097
The human toll signaling pathway: divergence of nuclear factor kappaB and JNK/SAPK activation upstream of tumor necrosis factor receptor-associated factor 6 (TRAF6)
Abstract
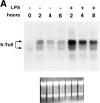
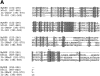
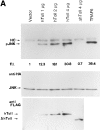
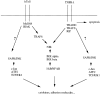
The human homologue of Drosophila Toll (hToll) is a recently cloned receptor of the interleukin 1 receptor (IL-1R) superfamily, and has been implicated in the activation of adaptive immunity. Signaling by hToll is shown to occur through sequential recruitment of the adapter molecule MyD88 and the IL-1R-associated kinase. Tumor necrosis factor receptor-activated factor 6 (TRAF6) and the nuclear factor kappaB (NF-kappaB)-inducing kinase (NIK) are both involved in subsequent steps of NF-kappaB activation. Conversely, a dominant negative version of TRAF6 failed to block hToll-induced activation of stress-activated protein kinase/c-Jun NH2-terminal kinases, thus suggesting an early divergence of the two pathways.
Figures





Similar articles
-
MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways.Mol Cell. 1998 Aug;2(2):253-8. doi: 10.1016/s1097-2765(00)80136-7. Mol Cell. 1998. PMID: 9734363
-
Toll-like receptor 3-mediated activation of NF-kappaB and IRF3 diverges at Toll-IL-1 receptor domain-containing adapter inducing IFN-beta.Proc Natl Acad Sci U S A. 2004 Mar 9;101(10):3533-8. doi: 10.1073/pnas.0308496101. Epub 2004 Feb 24. Proc Natl Acad Sci U S A. 2004. PMID: 14982987 Free PMC article.
-
Ras participates in CpG oligodeoxynucleotide signaling through association with toll-like receptor 9 and promotion of interleukin-1 receptor-associated kinase/tumor necrosis factor receptor-associated factor 6 complex formation in macrophages.J Biol Chem. 2003 Sep 19;278(38):36334-40. doi: 10.1074/jbc.M305698200. Epub 2003 Jul 16. J Biol Chem. 2003. PMID: 12867418
-
Innate immune induction of the adaptive immune response.Cold Spring Harb Symp Quant Biol. 1999;64:429-35. doi: 10.1101/sqb.1999.64.429. Cold Spring Harb Symp Quant Biol. 1999. PMID: 11232318 Review. No abstract available.
-
A universal role for MyD88 in TLR/IL-1R-mediated signaling.Trends Biochem Sci. 2002 Sep;27(9):474-82. doi: 10.1016/s0968-0004(02)02145-x. Trends Biochem Sci. 2002. PMID: 12217523 Review.
Cited by
-
A Standardized Chemically Modified Curcuma longa Extract Modulates IRAK-MAPK Signaling in Inflammation and Potentiates Cytotoxicity.Front Pharmacol. 2016 Jul 25;7:223. doi: 10.3389/fphar.2016.00223. eCollection 2016. Front Pharmacol. 2016. PMID: 27504095 Free PMC article.
-
MD-2, a novel accessory molecule, is involved in species-specific actions of Salmonella lipid A.Infect Immun. 2002 Jul;70(7):3546-50. doi: 10.1128/IAI.70.7.3546-3550.2002. Infect Immun. 2002. PMID: 12065494 Free PMC article.
-
Toll-like receptors in multiple sclerosis.Curr Top Microbiol Immunol. 2009;336:155-68. doi: 10.1007/978-3-642-00549-7_9. Curr Top Microbiol Immunol. 2009. PMID: 19688333 Free PMC article. Review.
-
The roll of Toll-like receptors in the antiphospholipid syndrome.Curr Rheumatol Rep. 2010 Feb;12(1):58-63. doi: 10.1007/s11926-009-0079-0. Curr Rheumatol Rep. 2010. PMID: 20425535 Review.
-
Pivotal role for neuronal Toll-like receptors in ischemic brain injury and functional deficits.Proc Natl Acad Sci U S A. 2007 Aug 21;104(34):13798-803. doi: 10.1073/pnas.0702553104. Epub 2007 Aug 10. Proc Natl Acad Sci U S A. 2007. PMID: 17693552 Free PMC article.
References
-
- Medzhitov R, Janeway A., Jr Innate immunity: the virtues of a nonclonal system of recognition. Cell. 1997;91:295–298. - PubMed
-
- Medzhitov R, Preston-Hurlburt P, Janeway CA. A human homologue of the DrosophilaToll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. - PubMed
-
- Lemaitre B, Nicolas E, Michaut L, Reichart J-M, Hoffmann JA. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophilaadults. Cell. 1996;86:973–983. - PubMed
-
- Greenfeder SA, Nunes P, Kwee L, Labow M, Chizzonite RA, Ju G. Molecular cloning and characterization of a second subunit of the interleukin 1 receptor complex. J Biol Chem. 1995;270:13757–13765. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

