Limited entry of adenovirus vectors into well-differentiated airway epithelium is responsible for inefficient gene transfer
- PMID: 9621064
- PMCID: PMC110406
- DOI: 10.1128/JVI.72.7.6014-6023.1998
Limited entry of adenovirus vectors into well-differentiated airway epithelium is responsible for inefficient gene transfer
Abstract
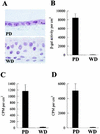
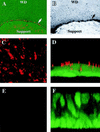
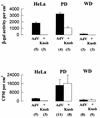
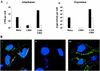
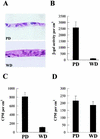
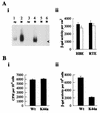
Investigations of the efficiency and safety of human adenovirus vector (AdV)-mediated gene transfer in the airways of patients with cystic fibrosis (CF) in vivo have demonstrated little success in correcting the CF bioelectrical functional defect, reflecting the inefficiency of AdV-mediated gene transfer to the epithelial cells that line the airway luminal surface. In this study, we demonstrate that low AdV-mediated gene transfer efficiency to well-differentiated (WD) cultured airway epithelial cells is due to three distinct steps in the apical membrane of the airway epithelial cells: (i) the absence of specific adenovirus fiber-knob protein attachment receptors; (ii) the absence of alphavbeta3/5 integrins, reported to partially mediate the internalization of AdV into the cell cytoplasm; and (iii) the low rate of apical plasma membrane uptake pathways of WD airway epithelial cells. Attempts to increase gene transfer efficiency by increasing nonspecific attachment of AdV were unsuccessful, reflecting the inability of the attached vector to enter (penetrate) WD cells via nonspecific entry paths. Strategies to improve the efficiency of AdV for the treatment of CF lung disease will require methods to increase the attachment of AdV to and promote its internalization into the WD respiratory epithelium.
Figures



Similar articles
-
Glycocalyx restricts adenoviral vector access to apical receptors expressed on respiratory epithelium in vitro and in vivo: role for tethered mucins as barriers to lumenal infection.J Virol. 2004 Dec;78(24):13755-68. doi: 10.1128/JVI.78.24.13755-13768.2004. J Virol. 2004. PMID: 15564484 Free PMC article.
-
Efficient adenovirus-mediated gene transfer to basal but not columnar cells of cartilaginous airway epithelia.Hum Gene Ther. 1996 May 20;7(8):921-31. doi: 10.1089/hum.1996.7.8-921. Hum Gene Ther. 1996. PMID: 8727506
-
Lack of high affinity fiber receptor activity explains the resistance of ciliated airway epithelia to adenovirus infection.J Clin Invest. 1997 Sep 1;100(5):1144-9. doi: 10.1172/JCI119625. J Clin Invest. 1997. PMID: 9276731 Free PMC article.
-
Adenovirus-mediated in vivo gene transfer.Ann N Y Acad Sci. 1994 May 31;716:90-101; discussion 101-3. doi: 10.1111/j.1749-6632.1994.tb21705.x. Ann N Y Acad Sci. 1994. PMID: 7517653 Review.
-
Adenovirus as a gene therapy vector for hematopoietic cells.Cancer Gene Ther. 2000 Jun;7(6):816-25. doi: 10.1038/sj.cgt.7700174. Cancer Gene Ther. 2000. PMID: 10880011 Review.
Cited by
-
Ferret and pig models of cystic fibrosis: prospects and promise for gene therapy.Hum Gene Ther Clin Dev. 2015 Mar;26(1):38-49. doi: 10.1089/humc.2014.154. Epub 2015 Feb 12. Hum Gene Ther Clin Dev. 2015. PMID: 25675143 Free PMC article. Review.
-
Bidirectional virus secretion and nonciliated cell tropism following Andes virus infection of primary airway epithelial cell cultures.J Virol. 2006 Feb;80(3):1087-97. doi: 10.1128/JVI.80.3.1087-1097.2006. J Virol. 2006. PMID: 16414986 Free PMC article.
-
Coxsackievirus and adenovirus receptor expression in non-malignant lung tissues and clinical lung cancers.J Mol Histol. 2006 May;37(3-4):153-60. doi: 10.1007/s10735-006-9055-4. Epub 2006 Sep 22. J Mol Histol. 2006. PMID: 17031523
-
Airway epithelial CFTR mRNA expression in cystic fibrosis patients after repetitive administration of a recombinant adenovirus.J Clin Invest. 1999 Nov;104(9):1245-55. doi: 10.1172/JCI7935. J Clin Invest. 1999. PMID: 10545523 Free PMC article. Clinical Trial.
-
Adenovirus type 37 uses sialic acid as a cellular receptor.J Virol. 2000 Jan;74(1):42-8. J Virol. 2000. PMID: 10590089 Free PMC article.
References
-
- Bergelson J M, Cunningham J A, Droguett G, Kurt-Jones E A, Krivithas A, Hong J S, Horowitz M S, Crowell R L, Finberg R W. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. - PubMed
-
- Boucher R C. Current status of CF gene therapy. Trends Genet. 1996;12:81–84. - PubMed
-
- Brighton C T, Albelda S M. Identification of cell substratum adhesion receptors of integrins on cultured rat bone cells. J Orthop Res. 1992;10:766–773. - PubMed
-
- Comstock K E, Watson N F, Olsen J C. Design of retroviral expression vectors. In: Tuan R, editor. Recombinant gene expression protocols. Totowa, N.J: Humana Press, Inc.; 1996. p. 207. - PubMed
-
- Crystal R G, Jaffe A, Brody S, Mastrangeli A, McElvaney N G, Rosenfeld M E, Chu C, Danel C, Hay J, Eissa T. A phase I study, in cystic fibrosis patients, of the safety, toxicity, and biological efficacy of a single administration of a replication-deficient, recombinant adenovirus carrying the cDNA of the normal cystic fibrosis transmembrane conductance regulator gene in the lung. Hum Gene Ther. 1995;6:643–666. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

