Role of the pre-S2 domain of the large envelope protein in hepatitis B virus assembly and infectivity
- PMID: 9621015
- PMCID: PMC110210
- DOI: 10.1128/JVI.72.7.5573-5578.1998
Role of the pre-S2 domain of the large envelope protein in hepatitis B virus assembly and infectivity
Abstract
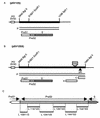
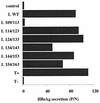
Among the three viral proteins present in the hepatitis B virus (HBV) envelope, both the small and large polypeptides, but not the middle polypeptide, are necessary for the production of complete viral particles. Whereas it has been established that the C-terminal extremity of the pre-S1 region is required for HBV morphogenesis, whether the pre-S2 region of the large surface protein plays a critical role remains questionable. In the present study, we have analyzed the role of the large-polypeptide pre-S2 region in viral maturation and infectivity. For this purpose, mutants bearing contiguous deletions covering the entire pre-S2 domain were generated. First, the efficient expression of all the mutant large envelope proteins was verified and their ability to substitute for the wild-type form in virion secretion was tested. We found that distinct deletions covering the domain between amino acids 114 and 163 still allowed virion production. In contrast, the polypeptide lacking the first 5 amino acids of pre-S2 (amino acids 109 to 113) was unable to support viral secretion. This result shows that the domain of the large surface protein, required for this process, must be extended to the N-terminal extremity of pre-S2. We then demonstrated that all the mutants competent for virion release were able to infect normal human hepatocytes in primary culture. Taken together, these results indicate that only 10% of the large-protein pre-S2 region at its N-terminal extremity is essential for virion export and that the remaining part, dispensable for viral secretion, is also dispensable for infectivity.
Figures


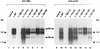

Similar articles
-
Infection process of the hepatitis B virus depends on the presence of a defined sequence in the pre-S1 domain.J Virol. 1999 Mar;73(3):2052-7. doi: 10.1128/JVI.73.3.2052-2057.1999. J Virol. 1999. PMID: 9971786 Free PMC article.
-
Mapping a region of the large envelope protein required for hepatitis B virion maturation.J Virol. 1994 Mar;68(3):1643-50. doi: 10.1128/JVI.68.3.1643-1650.1994. J Virol. 1994. PMID: 8107225 Free PMC article.
-
A short linear sequence in the pre-S domain of the large hepatitis B virus envelope protein required for virion formation.J Virol. 1997 Dec;71(12):9350-7. doi: 10.1128/JVI.71.12.9350-9357.1997. J Virol. 1997. PMID: 9371594 Free PMC article.
-
Functions of the large hepatitis B virus surface protein in viral particle morphogenesis.Intervirology. 1996;39(1-2):23-31. doi: 10.1159/000150471. Intervirology. 1996. PMID: 8957666 Review.
-
Methods for the study of pre-S proteins of hepatitis B virus and their antibodies: pathogenetic and clinical implications.Ric Clin Lab. 1988 Apr-Sep;18(2-3):241-58. doi: 10.1007/BF02918887. Ric Clin Lab. 1988. PMID: 3062749 Review.
Cited by
-
Efficient inhibition of hepatitis B virus infection by acylated peptides derived from the large viral surface protein.J Virol. 2005 Feb;79(3):1613-22. doi: 10.1128/JVI.79.3.1613-1622.2005. J Virol. 2005. PMID: 15650187 Free PMC article.
-
A short N-proximal region in the large envelope protein harbors a determinant that contributes to the species specificity of human hepatitis B virus.J Virol. 2001 Dec;75(23):11565-72. doi: 10.1128/JVI.75.23.11565-11572.2001. J Virol. 2001. PMID: 11689638 Free PMC article.
-
Infection process of the hepatitis B virus depends on the presence of a defined sequence in the pre-S1 domain.J Virol. 1999 Mar;73(3):2052-7. doi: 10.1128/JVI.73.3.2052-2057.1999. J Virol. 1999. PMID: 9971786 Free PMC article.
-
Formation of vesicular stomatitis virus pseudotypes bearing surface proteins of hepatitis B virus.J Virol. 2005 Oct;79(19):12566-74. doi: 10.1128/JVI.79.19.12566-12574.2005. J Virol. 2005. PMID: 16160184 Free PMC article.
-
The first transmembrane domain of the hepatitis B virus large envelope protein is crucial for infectivity.J Virol. 2009 Nov;83(22):11819-29. doi: 10.1128/JVI.01026-09. Epub 2009 Sep 9. J Virol. 2009. PMID: 19740987 Free PMC article.
References
-
- Aden D P, Fogel A, Plotkin S, Damjanov I, Knowles B B. Controlled synthesis of HBsAg in a differentiated human liver carcinoma-derived cell line. Nature (London) 1979;282:615–616. - PubMed
-
- Bruss V, Hagelsten J, Gerhardt E, Galle P R. Myristylation of the large surface protein is required for hepatitis B virus in vitro infectivity. Virology. 1996;218:396–399. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

