The histone acetylase PCAF is a nuclear receptor coactivator
- PMID: 9620851
- PMCID: PMC316869
- DOI: 10.1101/gad.12.11.1638
The histone acetylase PCAF is a nuclear receptor coactivator
Abstract
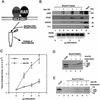
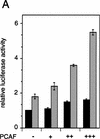
Whereas the histone acetylase PCAF has been suggested to be part of a coactivator complex mediating transcriptional activation by the nuclear hormone receptors, the physical and functional interactions between nuclear receptors and PCAF have remained unclear. Our efforts to clarify these relationships have revealed two novel properties of nuclear receptors. First, we demonstrate that the RXR/RAR heterodimer directly recruits PCAF from mammalian cell extracts in a ligand-dependent manner and that increased expression of PCAF leads to enhanced retinoid-responsive transcription. Second, we demonstrate that, in vitro, PCAF directly associates with the DNA-binding domain of nuclear receptors, independently of p300/CBP binding, therefore defining a novel cofactor interaction surface. Furthermore, our results show that dissociation of corepressors enables ligand-dependent PCAF binding to the receptors. This observation illuminates how a ligand-dependent receptor function can be propagated to regions outside the ligand-binding domain itself. On the basis of these observations, we suggest that PCAF may play a more central role in nuclear receptor function than previously anticipated.
Figures





















Similar articles
-
Molecular interaction of retinoic acid receptors with coregulators PCAF and RIP140.Mol Cell Endocrinol. 2004 Oct 29;226(1-2):43-50. doi: 10.1016/j.mce.2004.07.001. Mol Cell Endocrinol. 2004. PMID: 15489004
-
A regulatory role for RIP140 in nuclear receptor activation.Mol Endocrinol. 1998 Jun;12(6):864-81. doi: 10.1210/mend.12.6.0123. Mol Endocrinol. 1998. PMID: 9626662
-
Peroxisome proliferator-activated receptors and retinoic acid receptors differentially control the interactions of retinoid X receptor heterodimers with ligands, coactivators, and corepressors.Mol Cell Biol. 1997 Apr;17(4):2166-76. doi: 10.1128/MCB.17.4.2166. Mol Cell Biol. 1997. PMID: 9121466 Free PMC article.
-
Nuclear receptors, their coactivators and modulation of transcription.Acta Biochim Pol. 1999;46(1):77-89. Acta Biochim Pol. 1999. PMID: 10453983 Review.
-
Acetylation of general transcription factors by histone acetyltransferases.Curr Biol. 1997 Sep 1;7(9):689-92. doi: 10.1016/s0960-9822(06)00296-x. Curr Biol. 1997. PMID: 9285713 Review.
Cited by
-
Effects of PPARγ Ligands on Leukemia.PPAR Res. 2012;2012:483656. doi: 10.1155/2012/483656. Epub 2012 May 21. PPAR Res. 2012. PMID: 22685453 Free PMC article.
-
Analysis of the steroid receptor coactivator 1 (SRC1)-CREB binding protein interaction interface and its importance for the function of SRC1.Mol Cell Biol. 2001 Jan;21(1):39-50. doi: 10.1128/MCB.21.1.39-50.2001. Mol Cell Biol. 2001. PMID: 11113179 Free PMC article.
-
Structural and functional analysis of domains of the progesterone receptor.Mol Cell Endocrinol. 2012 Jan 30;348(2):418-29. doi: 10.1016/j.mce.2011.07.017. Epub 2011 Jul 22. Mol Cell Endocrinol. 2012. PMID: 21803119 Free PMC article. Review.
-
Vitamin D Switches BAF Complexes to Protect β Cells.Cell. 2018 May 17;173(5):1135-1149.e15. doi: 10.1016/j.cell.2018.04.013. Epub 2018 May 10. Cell. 2018. PMID: 29754817 Free PMC article.
-
MDM2 regulates estrogen receptor α and estrogen responsiveness in breast cancer cells.J Mol Endocrinol. 2011 Feb 15;46(2):67-79. doi: 10.1677/JME-10-0110. Print 2011 Apr. J Mol Endocrinol. 2011. PMID: 21169420 Free PMC article.
References
-
- Alland L, Muhle R, Hou Jr H, Potes J, Chin L, Schreiber-Agus N, DePinho RA. Role for N-CoR and histone deacetylase in Sin3-mediated transcriptional repression. Nature. 1997;387:49–55. - PubMed
-
- Bannister AJ, Kouzarides T. The CBP coactivator is a histone acetyltransferase. Nature. 1996;384:641–643. - PubMed
-
- Beato M. Gene regulation by steroid hormones. Cell. 1989;56:335–344. - PubMed
-
- Berger SL, Pina B, Silverman N, Marcus GA, Agapite J, Regier JL, Triezenberg SJ, Guarente L. Genetic isolation of ADA2: A potential transcriptional adaptor required for function of certain acidic activation domains. Cell. 1992;70:251–265. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
