Detection of a novel intraneuronal pool of insoluble amyloid beta protein that accumulates with time in culture
- PMID: 9585420
- PMCID: PMC2132781
- DOI: 10.1083/jcb.141.4.1031
Detection of a novel intraneuronal pool of insoluble amyloid beta protein that accumulates with time in culture
Abstract
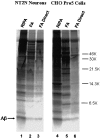
The amyloid-beta peptide (Abeta) is produced at several sites within cultured human NT2N neurons with Abeta1-42 specifically generated in the endoplasmic reticulum/intermediate compartment. Since Abeta is found as insoluble deposits in senile plaques of the AD brain, and the Abeta peptide can polymerize into insoluble fibrils in vitro, we examined the possibility that Abeta1-40, and particularly the more highly amyloidogenic Abeta1-42, accumulate in an insoluble pool within NT2N neurons. Remarkably, we found that formic acid extraction of the NT2N cells solubilized a pool of previously undetectable Abeta that accounted for over half of the total intracellular Abeta. Abeta1-42 was more abundant than Abeta1-40 in this pool, and most of the insoluble Abeta1-42 was generated in the endoplasmic reticulum/intermediate compartment pathway. High levels of insoluble Abeta were also detected in several nonneuronal cell lines engineered to overexpress the amyloid-beta precursor protein. This insoluble intracellular pool of Abeta was exceptionally stable, and accumulated in NT2N neurons in a time-dependent manner, increasing 12-fold over a 7-wk period in culture. These novel findings suggest that Abeta amyloidogenesis may be initiated within living neurons rather than in the extracellular space. Thus, the data presented here require a reexamination of the prevailing view about the pathogenesis of Abeta deposition in the AD brain.
Figures
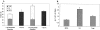



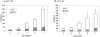
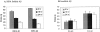
Similar articles
-
A distinct ER/IC gamma-secretase competes with the proteasome for cleavage of APP.Biochemistry. 2000 Feb 1;39(4):810-7. doi: 10.1021/bi991728z. Biochemistry. 2000. PMID: 10651647
-
Secretion and intracellular generation of truncated Abeta in beta-site amyloid-beta precursor protein-cleaving enzyme expressing human neurons.J Biol Chem. 2003 Feb 14;278(7):4458-66. doi: 10.1074/jbc.M210105200. Epub 2002 Dec 11. J Biol Chem. 2003. PMID: 12480937
-
Intracellular accumulation of insoluble, newly synthesized abetan-42 in amyloid precursor protein-transfected cells that have been treated with Abeta1-42.J Biol Chem. 1999 Jul 16;274(29):20650-6. doi: 10.1074/jbc.274.29.20650. J Biol Chem. 1999. PMID: 10400697
-
Alzheimer's disease.Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
-
[Involvement of beta-amyloid in the etiology of Alzheimer's disease].Brain Nerve. 2010 Jul;62(7):691-9. Brain Nerve. 2010. PMID: 20675873 Review. Japanese.
Cited by
-
Apolipoprotein E: from lipid transport to neurobiology.Prog Lipid Res. 2011 Jan;50(1):62-74. doi: 10.1016/j.plipres.2010.09.001. Epub 2010 Sep 18. Prog Lipid Res. 2011. PMID: 20854843 Free PMC article. Review.
-
Gradual alteration of mitochondrial structure and function by beta-amyloids: importance of membrane viscosity changes, energy deprivation, reactive oxygen species production, and cytochrome c release.J Bioenerg Biomembr. 2005 Aug;37(4):207-25. doi: 10.1007/s10863-005-6631-3. J Bioenerg Biomembr. 2005. PMID: 16167177
-
The Relationship between Parkin and Protein Aggregation in Neurodegenerative Diseases.Front Psychiatry. 2010 Jun 3;1:15. doi: 10.3389/fpsyt.2010.00015. eCollection 2010. Front Psychiatry. 2010. PMID: 21423426 Free PMC article.
-
Amyloid-beta protein clearance and degradation (ABCD) pathways and their role in Alzheimer's disease.Curr Alzheimer Res. 2015;12(1):32-46. doi: 10.2174/1567205012666141218140953. Curr Alzheimer Res. 2015. PMID: 25523424 Free PMC article.
-
A pH-dependent conformational transition of Abeta peptide and physicochemical properties of the conformers in the glial cell.Biochem J. 2002 Feb 1;361(Pt 3):547-56. doi: 10.1042/0264-6021:3610547. Biochem J. 2002. PMID: 11802784 Free PMC article.
References
-
- Borhelt DR, Thinakaran G, Eckman CB, Lee MK, Davenport F, Ratovitsky T, Prada CM, Kim G, Seekins S, Yager D, et al. Familial Alzheimer's disease-linked presenilin 1 variants elevate Abeta1-42/1-40 ratio in vitro and in vivo. Neuron. 1996;17:1005–1013. - PubMed
-
- Burdick D, Soreghan B, Kwon M, Kosmoski J, Knauer M, Henschen A, Yates J, Cotman C, Glabe C. Assembly and aggregation properties of synthetic Alzheimer's A4/beta amyloid peptide analogs. J Biol Chem. 1992;267:546–554. - PubMed
-
- Citron M, Oltersdorf T, Haass C, McConlogue L, Hung AY, Seubert P, Vigo-Pelfrey C, Lieberburg I, Selkoe DJ. Mutation of the beta-amyloid precursor protein in familial Alzheimer's disease increases beta-protein production. Nature. 1992;360:672–674. - PubMed
-
- Cook DG, Forman MS, Sung JC, Leight S, Kolson DL, Iwatsubo T, Lee VM-Y, Doms RW. Alzheimer's A beta(1-42) is generated in the endoplasmic reticulum/intermediate compartment of NT2N cells. Nat Med. 1997;3:1021–1023. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

