Ganglioside structure dictates signal transduction by cholera toxin and association with caveolae-like membrane domains in polarized epithelia
- PMID: 9585411
- PMCID: PMC2132772
- DOI: 10.1083/jcb.141.4.917
Ganglioside structure dictates signal transduction by cholera toxin and association with caveolae-like membrane domains in polarized epithelia
Abstract
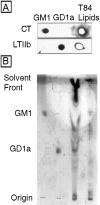
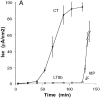
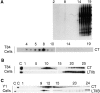
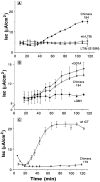
In polarized cells, signal transduction by cholera toxin (CT) requires apical endocytosis and retrograde transport into Golgi cisternae and perhaps ER (Lencer, W.I., C. Constable, S. Moe, M. Jobling, H.M. Webb, S. Ruston, J.L. Madara, T. Hirst, and R. Holmes. 1995. J. Cell Biol. 131:951-962). In this study, we tested whether CT's apical membrane receptor ganglioside GM1 acts specifically in toxin action. To do so, we used CT and the related Escherichia coli heat-labile type II enterotoxin LTIIb. CT and LTIIb distinguish between gangliosides GM1 and GD1a at the cell surface by virtue of their dissimilar receptor-binding B subunits. The enzymatically active A subunits, however, are homologous. While both toxins bound specifically to human intestinal T84 cells (Kd approximately 5 nM), only CT elicited a cAMP-dependent Cl- secretory response. LTIIb, however, was more potent than CT in eliciting a cAMP-dependent response from mouse Y1 adrenal cells (toxic dose 10 vs. 300 pg/well). In T84 cells, CT fractionated with caveolae-like detergent-insoluble membranes, but LTIIb did not. To investigate further the relationship between the specificity of ganglioside binding and partitioning into detergent-insoluble membranes and signal transduction, CT and LTIIb chimeric toxins were prepared. Analysis of these chimeric toxins confirmed that toxin-induced signal transduction depended critically on the specificity of ganglioside structure. The mechanism(s) by which ganglioside GM1 functions in signal transduction likely depends on coupling CT with caveolae or caveolae-related membrane domains.
Figures
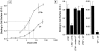

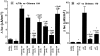
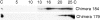
Similar articles
-
Characterization of receptor-mediated signal transduction by Escherichia coli type IIa heat-labile enterotoxin in the polarized human intestinal cell line T84.Infect Immun. 2001 Dec;69(12):7205-12. doi: 10.1128/IAI.69.12.7205-7212.2001. Infect Immun. 2001. PMID: 11705889 Free PMC article.
-
Targeting of cholera toxin and Escherichia coli heat labile toxin in polarized epithelia: role of COOH-terminal KDEL.J Cell Biol. 1995 Nov;131(4):951-62. doi: 10.1083/jcb.131.4.951. J Cell Biol. 1995. PMID: 7490296 Free PMC article.
-
Floating cholera toxin into epithelial cells: functional association with caveolae-like detergent-insoluble membrane microdomains.Int J Med Microbiol. 2000 Oct;290(4-5):403-8. doi: 10.1016/S1438-4221(00)80052-1. Int J Med Microbiol. 2000. PMID: 11111918 Review.
-
Membrane traffic and the cellular uptake of cholera toxin.Biochim Biophys Acta. 1999 Jul 8;1450(3):177-90. doi: 10.1016/s0167-4889(99)00070-1. Biochim Biophys Acta. 1999. PMID: 10395933 Review.
-
Filipin-dependent inhibition of cholera toxin: evidence for toxin internalization and activation through caveolae-like domains.J Cell Biol. 1998 May 18;141(4):905-15. doi: 10.1083/jcb.141.4.905. J Cell Biol. 1998. PMID: 9585410 Free PMC article.
Cited by
-
Lipid rafts act as specialized domains for tetanus toxin binding and internalization into neurons.Mol Biol Cell. 2001 Oct;12(10):2947-60. doi: 10.1091/mbc.12.10.2947. Mol Biol Cell. 2001. PMID: 11598183 Free PMC article.
-
Rafting with cholera toxin: endocytosis and trafficking from plasma membrane to ER.FEMS Microbiol Lett. 2007 Jan;266(2):129-37. doi: 10.1111/j.1574-6968.2006.00545.x. Epub 2006 Nov 29. FEMS Microbiol Lett. 2007. PMID: 17156122 Free PMC article. Review.
-
Clostridium difficile toxins disrupt epithelial barrier function by altering membrane microdomain localization of tight junction proteins.Infect Immun. 2001 Mar;69(3):1329-36. doi: 10.1128/IAI.69.3.1329-1336.2001. Infect Immun. 2001. PMID: 11179295 Free PMC article.
-
Intoxication of zebrafish and mammalian cells by cholera toxin depends on the flotillin/reggie proteins but not Derlin-1 or -2.J Clin Invest. 2010 Dec;120(12):4399-4409. doi: 10.1172/JCI42958. J Clin Invest. 2010. PMID: 21041954 Free PMC article.
-
Enterotoxin-based mucosal adjuvants alter antigen trafficking and induce inflammatory responses in the nasal tract.Infect Immun. 2005 Oct;73(10):6892-902. doi: 10.1128/IAI.73.10.6892-6902.2005. Infect Immun. 2005. PMID: 16177369 Free PMC article.
References
-
- Anderson RG. Plasmalemmal caveolae and GPI-anchored membrane proteins. Curr Opin Cell Biol. 1993b;5:647–652. - PubMed
-
- Anderson RG, Kamen BA, Rothberg KG, Lacey SW. Potocytosis: sequestration and transport of small molecules by caveolae. Science. 1992;255:410–411. - PubMed
-
- Bhatnagar RS, Gordon JI. Understanding covalent modifications of proteins by lipids: where cell biology and biophysics merge. Trends Cell Biol. 1997;7:14–20. - PubMed
-
- Bretscher MS, Munro S. Cholesterol and the Golgi apparatus. Science. 1993;261:1280–1281. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI031940/AI/NIAID NIH HHS/United States
- DK 48106/DK/NIDDK NIH HHS/United States
- R01 DK053056/DK/NIDDK NIH HHS/United States
- P01 DK033506/DK/NIDDK NIH HHS/United States
- R37 DK035932/DK/NIDDK NIH HHS/United States
- R01 DK048106/DK/NIDDK NIH HHS/United States
- DK/AI 53056/DK/NIDDK NIH HHS/United States
- P30 DK034854/DK/NIDDK NIH HHS/United States
- T32 DK007477/DK/NIDDK NIH HHS/United States
- T32-DK07477/DK/NIDDK NIH HHS/United States
- R01 AI014107/AI/NIAID NIH HHS/United States
- R37 DK048106/DK/NIDDK NIH HHS/United States
- R56 DK053056/DK/NIDDK NIH HHS/United States
- R01 AI053056/AI/NIAID NIH HHS/United States
- R37 AI014107/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

