Identification of kaposin (open reading frame K12) as a human herpesvirus 8 (Kaposi's sarcoma-associated herpesvirus) transforming gene
- PMID: 9573267
- PMCID: PMC110060
- DOI: 10.1128/JVI.72.6.4980-4988.1998
Identification of kaposin (open reading frame K12) as a human herpesvirus 8 (Kaposi's sarcoma-associated herpesvirus) transforming gene
Erratum in
- J Virol 1999 Mar;73(3):2568
Abstract
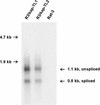
The recently identified human herpesvirus 8 (HHV-8, or Kaposi's sarcoma-associated herpesvirus) has been implicated in the etiology of both Kaposi's sarcoma (KS) and primary effusion (body cavity-based) lymphoma (PEL) (Y. Chang et al., Science 266:1865-1869, 1994; P. S. Moore et al., J. Virol. 70:549-558, 1996). An important feature of the association of HHV-8 with these malignancies is the expression of an abundant, latency-associated 0.7-kb transcript, T0. 7 (W. Zhong et al., Proc. Natl. Acad. Sci. USA 93:6641-6646, 1996). T0.7 is found in all stages in nearly all KS tumors of different epidemiologic origin, including AIDS-associated, African endemic, and classical KS (K. A. Staskus et al., J. Virol. 71:715-719, 1997), as well as in a body cavity-based lymphoma-derived cell line, BCBL-1, that is latently infected with HHV-8 (R. Renne et al., Nat. Med. 2:342-346, 1996). T0.7 encodes a unique HHV-8 open reading frame, K12, also known as kaposin. In this study, we report that the kaposin gene induced tumorigenic transformation. Constructs with kaposin expressed either from its endogenous promoter or from a heterologous promoter induced focal transformation upon transfection into Rat-3 cells. All transformed Rat-3 cell lines containing kaposin sequences produced high-grade, highly vascular, undifferentiated sarcomas upon subcutaneous injection of athymic nu/nu mice. Tumor-derived cell lines expressed kaposin mRNA, suggesting a role in the maintenance of the transformed phenotype. Furthermore, kaposin protein was detected in transformed and tumor-derived cells by immunofluorescence and localized to the cytoplasm. More importantly, expression of kaposin protein was also detected in the PEL cell lines BCBL-1 and KS-1. These findings demonstrate the oncogenic potential of kaposin and suggest its possible role in the development of KS and other HHV-8-associated malignancies.
Figures




Similar articles
-
Characterization of the human herpesvirus 8 (Kaposi's sarcoma-associated herpesvirus) oncogene, kaposin (ORF K12).J Clin Virol. 2000 May;16(3):203-13. doi: 10.1016/s1386-6532(99)00081-5. J Clin Virol. 2000. PMID: 10738139
-
A complex translational program generates multiple novel proteins from the latently expressed kaposin (K12) locus of Kaposi's sarcoma-associated herpesvirus.J Virol. 1999 Jul;73(7):5722-30. doi: 10.1128/JVI.73.7.5722-5730.1999. J Virol. 1999. PMID: 10364323 Free PMC article.
-
The 222- to 234-kilodalton latent nuclear protein (LNA) of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) is encoded by orf73 and is a component of the latency-associated nuclear antigen.J Virol. 1997 Aug;71(8):5915-21. doi: 10.1128/JVI.71.8.5915-5921.1997. J Virol. 1997. PMID: 9223481 Free PMC article.
-
KSHV Genome Replication and Maintenance in Latency.Adv Exp Med Biol. 2018;1045:299-320. doi: 10.1007/978-981-10-7230-7_14. Adv Exp Med Biol. 2018. PMID: 29896673 Review.
-
Human herpesvirus 8 (HHV-8/KSHV) and hematologic malignancies.Rev Clin Exp Hematol. 2003 Dec;7(4):375-405. Rev Clin Exp Hematol. 2003. PMID: 15129649 Review.
Cited by
-
Molecular genetics of Kaposi's sarcoma-associated herpesvirus (human herpesvirus-8) epidemiology and pathogenesis.Microbiol Mol Biol Rev. 2003 Jun;67(2):175-212, table of contents. doi: 10.1128/MMBR.67.2.175-212.2003. Microbiol Mol Biol Rev. 2003. PMID: 12794189 Free PMC article. Review.
-
KSHV: pathways to tumorigenesis and persistent infection.Adv Virus Res. 2014;88:111-59. doi: 10.1016/B978-0-12-800098-4.00002-7. Adv Virus Res. 2014. PMID: 24373311 Free PMC article. Review.
-
Kaposi's Sarcoma-Associated Herpesvirus Genome Replication, Partitioning, and Maintenance in Latency.Front Microbiol. 2012 Jan 24;3:7. doi: 10.3389/fmicb.2012.00007. eCollection 2012. Front Microbiol. 2012. PMID: 22291692 Free PMC article.
-
Human immunodeficiency virus type 1 Tat accelerates Kaposi sarcoma-associated herpesvirus Kaposin A-mediated tumorigenesis of transformed fibroblasts in vitro as well as in nude and immunocompetent mice.Neoplasia. 2009 Dec;11(12):1272-84. doi: 10.1593/neo.09494. Neoplasia. 2009. PMID: 20019835 Free PMC article.
-
Array-based transcript profiling and limiting-dilution reverse transcription-PCR analysis identify additional latent genes in Kaposi's sarcoma-associated herpesvirus.J Virol. 2010 Jun;84(11):5565-73. doi: 10.1128/JVI.02723-09. Epub 2010 Mar 10. J Virol. 2010. PMID: 20219929 Free PMC article.
References
-
- Adduci, A., et al. Unpublished data.
-
- Ambroziak J A, Blackbourn D J, Herndier B G, Glogau R G, Gullett J H, McDonald A R, Lennette E T, Levy J A. Herpes-like sequences in HIV-infected and uninfected Kaposi’s sarcoma patients. Science. 1995;268:582–583. - PubMed
-
- Arvanitakis L, Geras-Raaka E, Varma A, Gershengorn M C, Cesarman E. Human herpesvirus KSHV encodes a constitutively active G-protein-coupled receptor linked to cell proliferation. Nature. 1997;385:347–350. - PubMed
-
- Bais C, Santomasso B, Coso O, Arvanitakis L, Raaka E G, Gutkind J S, Asch A S, Cesarman E, Gerhengorn M C, Mesri E A. G-protein-coupled receptor of Kaposi’s sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator. Nature. 1998;391:86–89. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

