The juxtamembrane region of the cadherin cytoplasmic tail supports lateral clustering, adhesive strengthening, and interaction with p120ctn
- PMID: 9566976
- PMCID: PMC2132752
- DOI: 10.1083/jcb.141.3.779
The juxtamembrane region of the cadherin cytoplasmic tail supports lateral clustering, adhesive strengthening, and interaction with p120ctn
Abstract
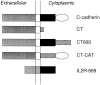
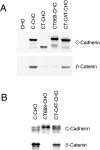
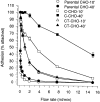
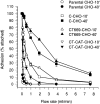
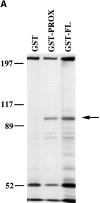
Cadherin cell-cell adhesion molecules form membrane-spanning molecular complexes that couple homophilic binding by the cadherin ectodomain to the actin cytoskeleton. A fundamental issue in cadherin biology is how this complex converts the weak intrinsic binding activity of the ectodomain into strong adhesion. Recently we demonstrated that cellular cadherins cluster in a ligand-dependent fashion when cells attached to substrata coated with the adhesive ectodomain of Xenopus C-cadherin (CEC1-5). Moreover, forced clustering of the ectodomain alone significantly strengthened adhesiveness (Yap, A.S., W.M. Brieher, M. Pruschy, and B.M. Gumbiner. Curr. Biol. 7:308-315). In this study we sought to identify the determinants of the cadherin cytoplasmic tail responsible for clustering activity. A deletion mutant of C-cadherin (CT669) that retained the juxtamembrane 94-amino acid region of the cytoplasmic tail, but not the beta-catenin-binding domain, clustered upon attachment to substrata coated with CEC1-5. Like wild-type C-cadherin, this clustering was ligand dependent. In contrast, mutant molecules lacking either the complete cytoplasmic tail or just the juxtamembrane region did not cluster. The juxtamembrane region was itself sufficient to induce clustering when fused to a heterologous membrane-anchored protein, albeit in a ligand-independent fashion. The CT669 cadherin mutant also displayed significant adhesive activity when tested in laminar flow detachment assays and aggregation assays. Purification of proteins binding to the juxtamembrane region revealed that the major associated protein is p120(ctn). These findings identify the juxtamembrane region of the cadherin cytoplasmic tail as a functionally active region supporting cadherin clustering and adhesive strength and raise the possibility that p120(ctn) is involved in clustering and cell adhesion.
Figures






Similar articles
-
An octapeptide in the juxtamembrane domain of VE-cadherin is important for p120ctn binding and cell proliferation.Exp Cell Res. 2002 Mar 10;274(1):35-44. doi: 10.1006/excr.2001.5436. Exp Cell Res. 2002. PMID: 11855855
-
Selective uncoupling of p120(ctn) from E-cadherin disrupts strong adhesion.J Cell Biol. 2000 Jan 10;148(1):189-202. doi: 10.1083/jcb.148.1.189. J Cell Biol. 2000. PMID: 10629228 Free PMC article.
-
Binding site for p120/delta-catenin is not required for Drosophila E-cadherin function in vivo.J Cell Biol. 2003 Feb 3;160(3):313-9. doi: 10.1083/jcb.200207160. Epub 2003 Jan 27. J Cell Biol. 2003. PMID: 12551956 Free PMC article.
-
Regulation of cadherin stability and turnover by p120ctn: implications in disease and cancer.Semin Cell Dev Biol. 2004 Dec;15(6):657-63. doi: 10.1016/j.semcdb.2004.09.003. Semin Cell Dev Biol. 2004. PMID: 15561585 Review.
-
Role of p120-catenin in cadherin trafficking.Biochim Biophys Acta. 2007 Jan;1773(1):8-16. doi: 10.1016/j.bbamcr.2006.07.005. Epub 2006 Jul 21. Biochim Biophys Acta. 2007. PMID: 16949165 Review.
Cited by
-
Cadherin cis and trans interactions are mutually cooperative.Proc Natl Acad Sci U S A. 2021 Mar 9;118(10):e2019845118. doi: 10.1073/pnas.2019845118. Proc Natl Acad Sci U S A. 2021. PMID: 33658369 Free PMC article.
-
N-terminal 1-54 amino acid sequence and Armadillo repeat domain are indispensable for P120-catenin isoform 1A in regulating E-cadherin.PLoS One. 2012;7(5):e37008. doi: 10.1371/journal.pone.0037008. Epub 2012 May 16. PLoS One. 2012. PMID: 22615871 Free PMC article.
-
Transendothelial migration of melanoma cells involves N-cadherin-mediated adhesion and activation of the beta-catenin signaling pathway.Mol Biol Cell. 2005 Sep;16(9):4386-97. doi: 10.1091/mbc.e05-03-0186. Epub 2005 Jun 29. Mol Biol Cell. 2005. PMID: 15987741 Free PMC article.
-
Listeria pathogenesis and molecular virulence determinants.Clin Microbiol Rev. 2001 Jul;14(3):584-640. doi: 10.1128/CMR.14.3.584-640.2001. Clin Microbiol Rev. 2001. PMID: 11432815 Free PMC article. Review.
-
The membrane-proximal region of the E-cadherin cytoplasmic domain prevents dimerization and negatively regulates adhesion activity.J Cell Biol. 1998 Sep 21;142(6):1605-13. doi: 10.1083/jcb.142.6.1605. J Cell Biol. 1998. PMID: 9744888 Free PMC article.
References
-
- Aberle H, Butz S, Stappert J, Weissig H, Kemler R, Hoschuetzky H. Assembly of the cadherin-catenin complex in vitro with recombinant proteins. J Cell Sci. 1994;107:3655–3663. - PubMed
-
- Chen H, Paradies NE, Fedor-Chaiken M, Brackenbury R. E-cadherin mediates adhesion and suppresses cell motility via distinct mechanisms. J Cell Sci. 1997;110:345–356. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

