Characterization of the human homologue of the yeast spc98p and its association with gamma-tubulin
- PMID: 9566969
- PMCID: PMC2132749
- DOI: 10.1083/jcb.141.3.689
Characterization of the human homologue of the yeast spc98p and its association with gamma-tubulin
Abstract
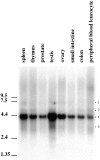
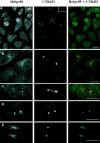
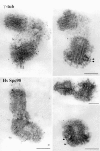
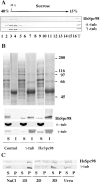
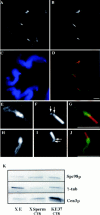
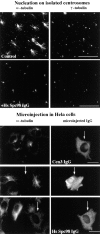
A trimeric complex formed by Tub4p, the budding yeast gamma-tubulin, and the two spindle pole body components, Spc98p and Spc97p, has recently been characterized in Saccharomyces cerevisiae. We reasoned that crucial functions, such as the control of microtubule nucleation, could be maintained among divergent species. SPC98-related sequences were searched in dbEST using the BLASTN program. Primers derived from the human expressed sequence tag matching SPC98 were used to clone the 5' and 3' cDNA ends by rapid amplification of cDNA ends (RACE)-PCR. The human Spc98 cDNA presents an alternative splicing at the 3' end. The deduced protein possesses 22% identity and 45% similarity with the yeast homologue. We further report that the human Spc98p, like gamma-tubulin, is concentrated at the centrosome, although a large fraction is found in cytosolic complexes. Sucrose gradient sedimentation of the cytosolic fraction and immunoprecipitation experiments demonstrate that both gamma-tubulin and HsSpc98p are in the same complex. Interestingly, Xenopus sperm centrosomes, which are incompetent for microtubule nucleation before their activation in the egg cytoplasm, were found to contain similar amounts of both Spc98p and gamma-tubulin to human somatic centrosomes, which are competent for microtubule nucleation. Finally, affinity-purified antibodies against Spc98p inhibit microtubule nucleation on isolated centrosomes, as well as in microinjected cells, suggesting that this novel protein is indeed required for the nucleation reaction.
Figures



Similar articles
-
The spindle pole body component Spc97p interacts with the gamma-tubulin of Saccharomyces cerevisiae and functions in microtubule organization and spindle pole body duplication.EMBO J. 1997 Apr 1;16(7):1550-64. doi: 10.1093/emboj/16.7.1550. EMBO J. 1997. PMID: 9130700 Free PMC article.
-
The spindle pole body component Spc98p interacts with the gamma-tubulin-like Tub4p of Saccharomyces cerevisiae at the sites of microtubule attachment.EMBO J. 1996 Aug 1;15(15):3899-911. EMBO J. 1996. PMID: 8670895 Free PMC article.
-
The mammalian gamma-tubulin complex contains homologues of the yeast spindle pole body components spc97p and spc98p.J Cell Biol. 1998 May 4;141(3):663-74. doi: 10.1083/jcb.141.3.663. J Cell Biol. 1998. PMID: 9566967 Free PMC article.
-
Microtubule organization by the budding yeast spindle pole body.Biol Cell. 1999 May-Jun;91(4-5):291-304. Biol Cell. 1999. PMID: 10518996 Review.
-
gamma-tubulin complexes: binding to the centrosome, regulation and microtubule nucleation.Curr Opin Cell Biol. 2000 Feb;12(1):113-8. doi: 10.1016/s0955-0674(99)00064-2. Curr Opin Cell Biol. 2000. PMID: 10679351 Review.
Cited by
-
Fission yeast MOZART1/Mzt1 is an essential γ-tubulin complex component required for complex recruitment to the microtubule organizing center, but not its assembly.Mol Biol Cell. 2013 Sep;24(18):2894-906. doi: 10.1091/mbc.E13-05-0235. Epub 2013 Jul 24. Mol Biol Cell. 2013. PMID: 23885124 Free PMC article.
-
GCP5 and GCP6: two new members of the human gamma-tubulin complex.Mol Biol Cell. 2001 Nov;12(11):3340-52. doi: 10.1091/mbc.12.11.3340. Mol Biol Cell. 2001. PMID: 11694571 Free PMC article.
-
BRCA1 is associated with the centrosome during mitosis.Proc Natl Acad Sci U S A. 1998 Oct 27;95(22):12983-8. doi: 10.1073/pnas.95.22.12983. Proc Natl Acad Sci U S A. 1998. PMID: 9789027 Free PMC article.
-
Protein 4.1 R-135 interacts with a novel centrosomal protein (CPAP) which is associated with the gamma-tubulin complex.Mol Cell Biol. 2000 Oct;20(20):7813-25. doi: 10.1128/MCB.20.20.7813-7825.2000. Mol Cell Biol. 2000. PMID: 11003675 Free PMC article.
-
Human 76p: A new member of the gamma-tubulin-associated protein family.J Cell Biol. 1999 Nov 15;147(4):857-68. doi: 10.1083/jcb.147.4.857. J Cell Biol. 1999. PMID: 10562286 Free PMC article.
References
-
- Archer J, Vega LR, Solomon F. Rbl2p, a yeast protein that binds to β-tubulin and participates in microtubule function in vivo. Cell. 1995;82:425–434. - PubMed
-
- Chrétien D, Buendia B, Fuller DF, Karsenti E. Reconstruction of the centrosome cycle from cryoelectron micrographs. J Struct Biol. 1997;120:117–133. - PubMed
-
- Détraves C, Mazarguil H, Lajoie-Mazenc I, Julian M, Raynaud-Messina B, Wright M. Protein complexes containing γ-tubulin are present in mammalian brain microtubule protein preparations. Cell Motil Cytoskel. 1997;36:179–189. - PubMed
-
- Dingwall, C., and R.A. Laskey. 1991. Nuclear targeting sequences—a consensus? Trends Biochem. Sci. 478–481. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

