Effectiveness of postinoculation (R)-9-(2-phosphonylmethoxypropyl) adenine treatment for prevention of persistent simian immunodeficiency virus SIVmne infection depends critically on timing of initiation and duration of treatment
- PMID: 9557716
- PMCID: PMC109656
- DOI: 10.1128/JVI.72.5.4265-4273.1998
Effectiveness of postinoculation (R)-9-(2-phosphonylmethoxypropyl) adenine treatment for prevention of persistent simian immunodeficiency virus SIVmne infection depends critically on timing of initiation and duration of treatment
Abstract
(R)-9-(2-Phosphonylmethoxypropyl)adenine (PMPA), an acyclic nucleoside phosphonate analog, is one of a new class of potent antiretroviral agents. Previously, we showed that PMPA treatment for 28 days prevented establishment of persistent simian immunodeficiency virus (SIV) infection in macaques even when therapy was initiated 24 h after intravenous virus inoculation. In the present study, we tested regimens involving different intervals between intravenous inoculation with SIV and initiation of PMPA treatment, as well as different durations of treatment, for the ability to prevent establishment of persistent infection. Twenty-four cynomolgus macaques (Macaca fascicularis) were studied for 46 weeks after inoculation with SIV. All mock-treated control macaques showed evidence of productive infection within 2 weeks postinoculation (p.i.). All macaques that were treated with PMPA for 28 days beginning 24 h p.i. showed no evidence of viral replication following discontinuation of PMPA treatment. However, extending the time to initiation of treatment from 24 to 48 or 72 h p.i. or decreasing the duration of treatment reduced effectiveness in preventing establishment of persistent infection. Only half of the macaques treated for 10 days, and none of those treated for 3 days, were completely protected when treatment was initiated at 24 h. Despite the reduced efficacy of delayed and shortened treatment, all PMPA-treated macaques that were not protected showed delays in the onset of cell-associated and plasma viremia and antibody responses compared with mock controls. These results clearly show that both the time between virus exposure and initiation of PMPA treatment as well as the duration of treatment are crucial factors for prevention of acute SIV infection in the macaque model.
Figures
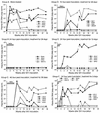
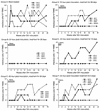

Similar articles
-
Prevention of SIV infection in macaques by (R)-9-(2-phosphonylmethoxypropyl)adenine.Science. 1995 Nov 17;270(5239):1197-9. doi: 10.1126/science.270.5239.1197. Science. 1995. PMID: 7502044
-
Administration of 9-[2-(phosphonomethoxy)propyl]adenine (PMPA) for prevention of perinatal simian immunodeficiency virus infection in rhesus macaques.AIDS Res Hum Retroviruses. 1998 Jun 10;14(9):761-73. doi: 10.1089/aid.1998.14.761. AIDS Res Hum Retroviruses. 1998. PMID: 9643376
-
Prophylactic and therapeutic benefits of short-term 9-[2-(R)-(phosphonomethoxy)propyl]adenine (PMPA) administration to newborn macaques following oral inoculation with simian immunodeficiency virus with reduced susceptibility to PMPA.J Virol. 2000 Feb;74(4):1767-74. doi: 10.1128/jvi.74.4.1767-1774.2000. J Virol. 2000. PMID: 10644348 Free PMC article.
-
Effects of (R)-9-(2-phosphonylmethoxypropyl)adenine monotherapy on chronic SIV infection in macaques.AIDS Res Hum Retroviruses. 1997 May 20;13(8):707-12. doi: 10.1089/aid.1997.13.707. AIDS Res Hum Retroviruses. 1997. PMID: 9168239
-
Two doses of PMPA protect newborn macaques against oral simian immunodeficiency virus infection.AIDS. 1998 Jun 18;12(9):F79-83. doi: 10.1097/00002030-199809000-00001. AIDS. 1998. PMID: 9662190
Cited by
-
Adherence, adverse drug reactions, and discontinuation associated with adverse drug reactions of HIV post-exposure prophylaxis: a meta-analysis based on cohort studies.Ann Med. 2023;55(2):2288309. doi: 10.1080/07853890.2023.2288309. Epub 2023 Dec 8. Ann Med. 2023. PMID: 38065681 Free PMC article.
-
A drug evaluation of 1% tenofovir gel and tenofovir disoproxil fumarate tablets for the prevention of HIV infection.Expert Opin Investig Drugs. 2012 May;21(5):695-715. doi: 10.1517/13543784.2012.667072. Epub 2012 Mar 7. Expert Opin Investig Drugs. 2012. PMID: 22394224 Free PMC article. Review.
-
HIV prevention in clinical care settings: 2014 recommendations of the International Antiviral Society-USA Panel.JAMA. 2014 Jul 23-30;312(4):390-409. doi: 10.1001/jama.2014.7999. JAMA. 2014. PMID: 25038358 Free PMC article.
-
Role of Fc-mediated antibody function in protective immunity against HIV-1.Immunology. 2014 May;142(1):46-57. doi: 10.1111/imm.12232. Immunology. 2014. PMID: 24843871 Free PMC article. Review.
-
Epigenetic Modulation of CD8⁺ T Cell Function in Lentivirus Infections: A Review.Viruses. 2018 Apr 28;10(5):227. doi: 10.3390/v10050227. Viruses. 2018. PMID: 29710792 Free PMC article. Review.
References
-
- Balzarini J, Holy A, Jindrich J, Naesens L, Snoeck R, Schols D, DeClerq E. Differential antiherpesvirus and antiretrovirus effects of the (S) and (R) enantiomers of acyclic nucleoside phosphonates: potent and selective in vitro and in vivo antiretrovirus activities of (R)-9-(-2-phosphonomethoxypropyl)-2,6-diaminopurine. Antimicrob Agents Chemother. 1993;37:332–338. - PMC - PubMed
-
- Benveniste R E, Morton W R, Clark E A, Tsai C-C, Ochs H D, Ward J M, Kuller L, Knott W B, Hill R W, Gale M J, Thouless M E. Inoculation of baboons and macaques with simian immunodeficiency virus/mne, a primate lentivirus closely related to human immunodeficiency virus type 2. J Virol. 1988;62:2091–2101. - PMC - PubMed
-
- Bertolli J, St. Louis M E, Simonds R J, Nieburg P, Kamenga M, Brown C, Tarande M, Quinn T, Ou C Y. Estimating the timing of mother-to-child transmission of human immunodeficiency virus in a breast-feeding population in Kinshasa, Zaire. J Infect Dis. 1996;174:722–726. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

